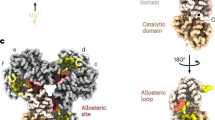
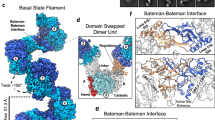
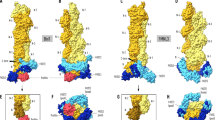
Abstract
Many enzymes assemble into defined oligomers, providing a mechanism for cooperatively regulating activity. Recent studies have described a mode of regulation in which enzyme activity is modulated by polymerization into large-scale filaments. Here we describe an ultrasensitive form of polymerization-based regulation employed by human CTP synthase 2 (CTPS2). Cryo-EM structures reveal that CTPS2 filaments dynamically switch between active and inactive forms in response to changes in substrate and product levels. Linking the conformational state of many CTPS2 subunits in a filament results in highly cooperative regulation, greatly exceeding the limits of cooperativity for the CTPS2 tetramer alone. The structures reveal a link between conformation and control of ammonia channeling between the enzyme’s active sites, and explain differences in regulation of human CTPS isoforms. This filament-based mechanism of enhanced cooperativity demonstrates how the widespread phenomenon of enzyme polymerization can be adapted to achieve different regulatory outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Trudel, M., Van Genechten, T. & Meuth, M. Biochemical characterization of the hamster thy mutator gene and its revertants. J. Biol. Chem. 259, 2355–2359 (1984).
Whelan, J., Phear, G., Yamauchi, M. & Meuth, M. Clustered base substitutions in CTP synthetase conferring drug resistance in Chinese hamster ovary cells. Nat. Genet. 3, 317–322 (1993).
Aronow, B., Watts, T., Lassetter, J., Washtien, W. & Ullman, B. Biochemical phenotype of 5-fluorouracil-resistant murine T-lymphoblasts with genetically altered CTP synthetase activity. J. Biol. Chem. 259, 9035–9043 (1984).
Carman, G. M. & Zeimetz, G. M. Regulation of phospholipid biosynthesis in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 271, 13293–13296 (1996).
Endrizzi, J. A., Kim, H., Anderson, P. M. & Baldwin, E. P. Mechanisms of product feedback regulation and drug resistance in cytidine triphosphate synthetases from the structure of a CTP-inhibited complex. Biochemistry 44, 13491–13499 (2005).
Habrian, C. et al. Inhibition of Escherichia coli CTP synthetase by NADH and other nicotinamides and their mutual interactions with CTP and GTP. Biochemistry 55, 5554–5565 (2016).
Goto, M., Omi, R., Nakagawa, N., Miyahara, I. & Hirotsu, K. Crystal structures of CTP synthetase reveal ATP, UTP, and glutamine binding sites. Structure 12, 1413–1423 (2004).
MacDonnell, J. E., Lunn, F. A. & Bearne, S. L. Inhibition of E. coli CTP synthase by the ‘positive’ allosteric effector GTP. Biochim. Biophys. Acta 1699, 213–220 (2004).
Endrizzi, J. A., Kim, H., Anderson, P. M. & Baldwin, E. P. Crystal structure of Escherichia coli cytidine triphosphate synthetase, a nucleotide-regulated glutamine amidotransferase/ATP-dependent amidoligase fusion protein and homologue of anticancer and antiparasitic drug targets. Biochemistry 43, 6447–6463 (2004).
Lynch, E. M. et al. Human CTP synthase filament structure reveals the active enzyme conformation. Nat. Struct. Mol. Biol. 24, 507–514 (2017).
Martin, E. et al. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature 510, 288–292 (2014).
Kucuk, Z. Y., Zhang, K., Filipovich, L. & Bleesing, J. J. CTP synthase 1 deficiency in successfully transplanted siblings with combined immune deficiency and chronic active EBV infection. J. Clin. Immunol. 36, 750–753 (2016).
Williams, J. C., Kizaki, H., Weber, G. & Morris, H. P. Increased CTP synthetase activity in cancer cells. Nature 271, 71–73 (1978).
Fan, H., Lu, S., Wang, S. & Zhang, S. Identification of critical genes associated with human osteosarcoma metastasis based on integrated gene expression profiling. Mol. Med. Rep. 20, 915–930 (2019).
Barry, R. M. et al. Large-scale filament formation inhibits the activity of CTP synthetase. eLife 3, e03638 (2014).
Anthony, S. A. et al. Reconstituted IMPDH polymers accommodate both catalytically active and inactive conformations. Mol. Biol. Cell 28, 2589–2745 (2017).
Webb, B. A., Dosey, A. M., Wittmann, T., Kollman, J. M. & Barber, D. L. The glycolytic enzyme phosphofructokinase-1 assembles into filaments. J. Cell Biol. 216, 2305–2313 (2017).
Hunkeler, M. et al. Structural basis for regulation of human acetyl-CoA carboxylase. Nature 558, 470–474 (2018).
Carcamo, W. C. et al. Induction of cytoplasmic rods and rings structures by inhibition of the CTP and GTP synthetic pathway in mammalian cells. PloS ONE 6, e29690 (2011).
Strochlic, T. I. et al. Ack kinase regulates CTP synthase filaments during Drosophila oogenesis. EMBO Rep. 15, 1184–1191 (2014).
Petrovska, I. et al. Filament formation by metabolic enzymes is a specific adaptation to an advanced state of cellular starvation. eLife 3, e02409 (2014).
Aughey, G. N. et al. Nucleotide synthesis is regulated by cytoophidium formation during neurodevelopment and adaptive metabolism. Biol. Open 3, 1045–1056 (2014).
Calise, S. J. et al. Glutamine deprivation initiates reversible assembly of mammalian rods and rings. Cell Mol. Life Sci. 71, 2963–2973 (2014).
McCluskey, G. D. & Bearne, S. L. ‘Pinching’ the ammonia tunnel of CTP synthase unveils coordinated catalytic and allosteric-dependent control of ammonia passage. Biochim. Biophys. Acta 1862, 2714–2727 (2018).
Willemoes, M. & Sigurskjold, B. W. Steady-state kinetics of the glutaminase reaction of CTP synthase from Lactococcus lactis. Eur. J. Biochem. 269, 4772–4779 (2002).
Kang, G. J. et al. Cyclopentenylcytosine triphosphate. Formation and inhibition of CTP synthetase. J. Biol. Chem. 264, 713–718 (1989).
Long, C. W. & Pardee, A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J. Biol. Chem. 242, 4715–4721 (1967).
McCluskey, G. D., Mohamady, S., Taylor, S. D. & Bearne, S. L. Exploring the potent inhibition of CTP synthase by gemcitabine-5′-triphosphate. Chembiochem 17, 2240–2249 (2016).
Kassel, K. M., Au da, R., Higgins, M. J., Hines, M. & Graves, L. M. Regulation of human cytidine triphosphate synthetase 2 by phosphorylation. J. Biol. Chem. 285, 33727–33736 (2010).
Choi, M. G. & Carman, G. M. Phosphorylation of human CTP synthetase 1 by protein kinase A: identification of Thr455 as a major site of phosphorylation. J. Biol. Chem. 282, 5367–5377 (2007).
Bray, D. & Duke, T. Conformational spread: the propagation of allosteric states in large multiprotein complexes. Annu. Rev. Biophys. Biomol. Struct. 33, 53–73 (2004).
Wyman, J. Possible allosteric effects in extended biological systems. J. Mol. Biol. 39, 523–538 (1969).
Changeux, J. P., Thiery, J., Tung, Y. & Kittel, C. On the cooperativity of biological membranes. Proc. Natl Acad. Sci. USA 57, 335–341 (1967).
Sourjik, V. & Berg, H. C. Functional interactions between receptors in bacterial chemotaxis. Nature 428, 437–441 (2004).
Bai, F. et al. Conformational spread as a mechanism for cooperativity in the bacterial flagellar switch. Science 327, 685–689 (2010).
Cluzel, P., Surette, M. & Leibler, S. An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Science 287, 1652–1655 (2000).
Marx, S. O., Ondrias, K. & Marks, A. R. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science 281, 818–821 (1998).
Meuth, M. The molecular basis of mutations induced by deoxyribonucleoside triphosphate pool imbalances in mammalian cells. Exp. Cell Res. 181, 305–316 (1989).
Mathews, C. K. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat. Rev. Cancer 15, 528–539 (2015).
Noree, C., Sato, B. K., Broyer, R. M. & Wilhelm, J. E. Identification of novel filament-forming proteins in Saccharomyces cerevisiae and Drosophila melanogaster. J. Cell Biol. 190, 541–551 (2010).
Liu, J. L. Intracellular compartmentation of CTP synthase in Drosophila. J. Genet. Genomics 37, 281–296 (2010).
Ingerson-Mahar, M., Briegel, A., Werner, J. N., Jensen, G. J. & Gitai, Z. The metabolic enzyme CTP synthase forms cytoskeletal filaments. Nat. Cell Biol. 12, 739–746 (2010).
Shen, Q. J. et al. Filamentation of metabolic enzymes in Saccharomyces cerevisiae. J. Genet. Genomics 43, 393–404 (2016).
Han, G. S. et al. Expression of human CTP synthetase in Saccharomyces cerevisiae reveals phosphorylation by protein kinase A. J. Biol. Chem. 280, 38328–38336 (2005).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Egelman, E. H. The iterative helical real space reconstruction method: surmounting the problems posed by real polymers. J. Struct. Biol. 157, 83–94 (2007).
Sachse, C. et al. High-resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus. J. Mol. Biol. 371, 812–835 (2007).
Egelman, E. H. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy 85, 225–234 (2000).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Grant, T., Rohou, A. & Grigorieff, N. cisTEM, user-friendly software for single-particle image processing. eLife 7, e35383 (2018).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Lander, G. C. et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J. Struct. Biol. 166, 95–102 (2009).
Webb, B. & Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci. 86, 2.9.1–2.9.37 (2016).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Jurcik, A. et al. CAVER Analyst 2.0: analysis and visualization of channels and tunnels in protein structures and molecular dynamics trajectories. Bioinformatics 34, 3586–3588 (2018).
Sakamoto, K. et al. Identification of cytidine-5-triphosphate synthase1-selective inhibitory peptide from random peptide library displayed on T7 phage. Peptides 94, 56–63 (2017).
Acknowledgements
We thank G. Carman (Rutgers University) for the CTPS-expressing S. cerevisiae strains. We thank E. Baldwin (UC Davis), who provided valuable feedback on our experimental results and early drafts of this manuscript. We are grateful to the Arnold and Mabel Beckman Cryo-EM Center at the University of Washington for use of electron microscopes. This work was supported by the US National Institutes of Health (grant no. R01 GM118396 to J.M.K.).
Author information
Authors and Affiliations
Contributions
E.M.L. performed the experiments. E.M.L. and J.M.K. designed experiments, performed data analysis and interpretation and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Inês Chen was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 CryoEM data processing flowchart for the S-state CTPS2 filament.
Additional details are provided in the Methods section.
Extended Data Fig. 2 CryoEM data processing flowchart for the P-state CTPS2 filament.
Additional details are provided in the Methods section.
Extended Data Fig. 3 Details of CTPS2 cryoEM structures.
a,b FSC curves for the S-state (a) and P-state (b) CTPS2 filament structures, showing resolutions of 3.5Å and 3.1Å, respectively, by the FSC0.143 criteria. c,d, ResMap local resolution maps of the S-state (c) and P-state (d) CTPS2 filament structures. e, CTPS2 tetramer, with monomers A-D shown in different colors. f, Zoomed-in view of the orange box in (e), showing S-state CTPS2 (blue), P-state CTPS2 (dark grey), and S-state CTPS1 (light grey) filament structures aligned on the amidoligase domain of subunit A. S-state CTPS1 and CTPS2 are extended across the tetramer interface, compared to P-state CTPS2. g, Monomers from the S-state (green) and P-state (dark grey) CTPS2 filament structures aligned on the amidoligase domain. h, Zoomed-in view of the blue box in (g), with S-State CTPS1 also shown in light grey. The glutaminase domain is rotated by 7o towards the amidoligase domain in the S-state structure. i,j Two views of the S-state (color) and P-state (grey) CTPS2 monomers aligned on the glutaminase-amidoligase interface. k, Zoomed-in view of the yellow box in (i). l, Zoomed-in view of the orange box in (j). The glutaminase-amidoligase interface is essentially identical (Cα RMSD 0.8 Å) in the S-state and P-state filaments.
Extended Data Fig. 4 Rare apo CTPS2 filaments have S-state filament architecture.
a, Negative stain EM images of apo CTPS2. Occasional filaments are observed. b, Helical rise (red) and rotation (black) values plotted over multiple rounds of iterative helical real space reconstruction of apo CTPS2 filaments in stain. Starting helical symmetry values and models from cryoEM structures of S-state (closed circles) or P-state (open circles) CTPS2 filaments were used. Both reconstructions converge on the S-state filament helical symmetry values. c, The apo CTPS2 filament structures from the reconstructions described in (b) are the same and have the S-state helical rotation, regardless of which starting symmetries and models are used. Scale bars are 50 nm.
Extended Data Fig. 5 Product-bound CTPS1 tetramers occasionally associate.
a, Negative stain EM images of CTPS1 in the presence of CTP and ADP. b, Zoomed-in view of the blue box in (a). The majority of CTPS1 is tetrameric, but rare pairs of tetramers are also observed (orange box). c, Reference-free 2D averages of CTPS1 in the presence of CTP and ADP. The percentage of all particles in each class is indicated. A single class containing pairs of tetramers (orange box) accounts for 3% of all particles. d, Negative stain EM reconstruction of the product-bound CTPS1 tetramer at 18Å resolution. The overall architecture resembles structures of existing CTPS tetramers. A CTPS1 tetramer from PBD 5U03 is fit for comparison. Scale bars are 50 nm.
Extended Data Fig. 6 The CTPS1 C-terminus is required for filament formation.
a, CryoEM maps of CTPS1 and CTPS2 filaments, low-pass filtered to 8Å for comparison. CTPS1 filaments have an additional C-terminal filament contact (orange) that is not observed in S- or P-state CTPS2 filaments. In the CTPS2 filament structures, the distance between adjacent C-termini increases by 8Å upon transition from the S-state to the P-state conformation. b, Sequence alignment of the CTPS1 and CTPS2 C-termini. Conserved residues are shown in blue. The C-termini have 41% sequence identity, compared with 75% overall sequence identity. c, Negative stain EM images of CTPS2-∆C, wild-type CTPS1, and CTPS1-∆C in the presence of substrates or products. CTPS1-∆C does not form filaments. d, Model for the role of the C-terminus in CTPS1 and CTPS2 polymerization. The CTPS1 C-terminus forms a contact that stabilizes the S-state filament, but is incompatible with the P-state conformation. The CTPS2 C-terminus is not required for polymerization. The lack of density for the CTPS2 C-terminus in the S- and P-state structures suggests it is disordered. e, Sequence alignment of CTPS1 and CTPS2 primary filament contacts, with differing residues highlighted in red. f,g CTPS1 (f) and CTPS2 (g) primary filament contact. Residues 347 and 354 adjacent to conserved H355, which differ between the two structures, are shown in orange and yellow. Scale bars are 50 nm.
Extended Data Fig. 7 CryoEM density at the P52-V58 and H55 constriction points.
a,b CryoEM density (grey) and atomic models (color) at the H55 gate in the P-state (a) and S-state (b) CTPS2 filaments. c,d CryoEM density (grey) and atomic models (color) at the P52-V58 constriction in the P-state (c) and S-state (d) CTPS2 filaments.
Extended Data Fig. 8 CTPS2cc forms disulfide crosslinks.
SDS-PAGE gel of wild-type CTPS2 and CTPS2CC under reducing (100 mM DTT) and non-reducing conditions. Bands for crosslinked CTPS2CC are visible under non-reducing conditions.
Extended Data Fig. 9 ADP-Glo assays show a linear response in relative light units (RLU) over the time used for CTPS2 kinetics assays.
a, Time-course of ADP-Glo assay under the substrate conditions used for CTP inhibition assays at 300 nM CTPS2 (open circles) and 1500 nM CTPS2 (closed circles). Rates were calculated from 12 minute timepoints for 1500 nM CTPS2 and 60 minutes for 300 nM CTPS2. b, Time-course of ADP-Glo assay at 1500 nM CTPS2 with the lowest UTP concentration used in UTP kinetics assays (10 µM UTP). Dashed lines indicate the linear portion of the assay used to measure reaction velocity. Data shown are mean and s.d. of n = 3 technical replicates for all panels.
Supplementary information
Supplementary Information
Supplementary Table 1.
Supplementary Video 1
Animation of the CTPS2 active site morphing between the S-state and P-state conformations. The glutaminase domain (green) rotates towards the amidoligase domain (blue), while loop P52–V58 (orange) is pulled towards the UTP base (magenta) in the S-state. ATP (yellow) is also shown in the active site.
Supplementary Video 2
Animation of CTPS2 morphing between the S-state and P-state filament conformations. Colored by tetramer. Filament contact sites (orange) remain the same despite conformational changes between the two filament states.
Rights and permissions
About this article
Cite this article
Lynch, E.M., Kollman, J.M. Coupled structural transitions enable highly cooperative regulation of human CTPS2 filaments. Nat Struct Mol Biol 27, 42–48 (2020). https://doi.org/10.1038/s41594-019-0352-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-019-0352-5
This article is cited by
-
CTP synthase 2 predicts inferior survival and mediates DNA damage response via interacting with BRCA1 in chronic lymphocytic leukemia
Experimental Hematology & Oncology (2023)
-
Structural basis of human PRPS2 filaments
Cell & Bioscience (2023)
-
Human PRPS1 filaments stabilize allosteric sites to regulate activity
Nature Structural & Molecular Biology (2023)
-
Agglomeration: when folded proteins clump together
Biophysical Reviews (2023)
-
IMPDH1 retinal variants control filament architecture to tune allosteric regulation
Nature Structural & Molecular Biology (2022)