Abstract
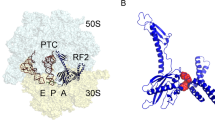
The drug-like molecule PF-06446846 (PF846) binds the human ribosome and selectively blocks the translation of a small number of proteins by an unknown mechanism. In structures of PF846-stalled human ribosome nascent chain complexes, PF846 binds in the ribosome exit tunnel in a eukaryotic-specific pocket formed by 28S ribosomal RNA, and alters the path of the nascent polypeptide chain. PF846 arrests the translating ribosome in the rotated state of translocation, in which the peptidyl-transfer RNA 3′-CCA end is improperly docked in the peptidyl transferase center. Selections of messenger RNAs from mRNA libraries using translation extracts reveal that PF846 can stall translation elongation, arrest termination or even enhance translation, depending on nascent chain sequence context. These results illuminate how a small molecule selectively targets translation by the human ribosome, and provides a foundation for developing small molecules that modulate the production of proteins of therapeutic interest.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The cryo-EM maps have been deposited with the Electron Microscopy Data Bank under the accession codes EMD-0599 (CDH1-RNC with A/A and P/E tRNA), EMD-0600 (CDH1-RNC with A/P and P/E tRNA), EMD-0601 (CDH1-RNC with P/P tRNA), EMD-0597 (PCSK9-RNC with A/A and P/E tRNA), EMD-0596 (PCSK9-RNC with A/P and P/E tRNA), EMD-0598 (PCSK9-RNC with P/P tRNA), EMD-20134 (PCSK9-RNC in rotated state with sample prepared with a short sample incubation time), EMD-20135 (PCSK9-RNC in non-rotated state with sample prepared with a short sample incubation time) and EMD-0526 (USO1-RNC with A/P and P/E tRNA). Atomic Coordinates have been deposited in the Protein Data Bank with accession codes 6OLF (CDH1-RNC with A/A and P/E tRNA), 6OLE (CDH1-RNC with A/P and P/E tRNA), 6OLG (CDH1-RNC with P/P tRNA), 6OM7 (PCSK9-RNC with A/A and P/E tRNA), 6OM0 (PCSK9-RNC with A/P and P/E tRNA), 6OLZ (PCSK9-RNC with P/P tRNA) and 6OLI (USO1-RNC with A/P and P/E tRNA). Sequencing data is available at the Sequence Read Archive (SRA) under accession (PRJNA533782). Analysis of all reads from the sequencing data for the PTC library and PF library is available in Supplementary Data Set 2. Source data for Figs. 1c and 5b,e,f and Supplementary Figs. 1a, 9 and 10c are available in Supplementary Data Set 3. All other data that support the findings of this study are available from the corresponding author upon reasonable request. The uncropped images for the main text are available in Supplementary Data Set 1.
References
Lin, J., Zhou, D., Steitz, T. A., Polikanov, Y. S. & Gagnon, M. G. Ribosome-targeting antibiotics: modes of action, mechanisms of resistance, and implications for drug design. Annu. Rev. Biochem. 87, 451–478 (2018).
Rinehart, K. L. Jr et al. Didemnins: antiviral and antitumor depsipeptides from a Caribbean tunicate. Science 212, 933–935 (1981).
Myasnikov, A. G. et al. Structure-function insights reveal the human ribosome as a cancer target for antibiotics. Nat. Commun. 7, 12856 (2016).
Almutairi, M. M. et al. Co-produced natural ketolides methymycin and pikromycin inhibit bacterial growth by preventing synthesis of a limited number of proteins. Nucleic Acids Res. 45, 9573–9582 (2017).
Kannan, K. & Mankin, A. S. Macrolide antibiotics in the ribosome exit tunnel: species-specific binding and action. Ann. N.Y. Acad. Sci. 1241, 33–47 (2011).
Wilson, D. N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 12, 35–48 (2014).
Garreau de Loubresse, N. et al. Structural basis for the inhibition of the eukaryotic ribosome. Nature 513, 517–522 (2014).
Marks, J. et al. Context-specific inhibition of translation by ribosomal antibiotics targeting the peptidyl transferase center. Proc. Natl Acad. Sci. USA 113, 12150–12155 (2016).
Blanchard, S. C., Cooperman, B. S. & Wilson, D. N. Probing translation with small-molecule inhibitors. Chem. Biol. 17, 633–645 (2010).
Lintner, N. G. et al. Selective stalling of human translation through small-molecule engagement of the ribosome nascent chain. PLoS Biol. 15, e2001882 (2017).
Londregan, A. T. et al. Small molecule proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: hit to lead optimization of systemic agents. J. Med. Chem. 61, 5704–5718 (2018).
Liaud, N. et al. Cellular response to small molecules that selectively stall protein synthesis by the ribosome. PLOS Genet. 15, e1008057 (2019).
Petersen, D. N. et al. A small-molecule anti-secretagogue of PCSK9 targets the 80S ribosome to inhibit PCSK9 protein translation. Cell Chem. Biol. 23, 1362–1371 (2016).
Hirano, S., Nose, A., Hatta, K., Kawakami, A. & Takeichi, M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J. Cell Biol. 105, 2501–2510 (1987).
Bhushan, S. et al. Alpha-helical nascent polypeptide chains visualized within distinct regions of the ribosomal exit tunnel. Nat. Struct. Mol. Biol. 17, 313–317 (2010).
Noller, H. F., Lancaster, L., Mohan, S. & Zhou, J. Ribosome structural dynamics in translocation: yet another functional role for ribosomal RNA. Q. Rev. Biophys. 50, e12 (2017).
Cannone, J. J. et al. The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics 3, 2 (2002).
Wilson, D. N. & Beckmann, R. The ribosomal tunnel as a functional environment for nascent polypeptide folding and translational stalling. Curr. Opin. Struct. Biol. 21, 274–282 (2011).
Su, T. et al. The force-sensing peptide VemP employs extreme compaction and secondary structure formation to induce ribosomal stalling. eLife 6, e25642 (2017).
Matheisl, S., Berninghausen, O., Becker, T. & Beckmann, R. Structure of a human translation termination complex. Nucleic Acids Res 43, 8615–8626 (2015).
Voorhees, R. M. & Hegde, R. S. Structures of the scanning and engaged states of the mammalian SRP-ribosome complex. eLife 4, e07975 (2015).
Dever, T. E. & Green, R. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harb. Perspect. Biol. 4, a013706 (2012).
Wasserman, M. R., Alejo, J. L., Altman, R. B. & Blanchard, S. C. Multiperspective smFRET reveals rate-determining late intermediates of ribosomal translocation. Nat. Struct. Mol. Biol. 23, 333–341 (2016).
Belardinelli, R. et al. Choreography of molecular movements during ribosome progression along mRNA. Nat. Struct. Mol. Biol. 23, 342–348 (2016).
Wang, L., Altman, R. B. & Blanchard, S. C. Insights into the molecular determinants of EF-G catalyzed translocation. RNA 17, 2189–2200 (2011).
Li, R., Kang, G., Hu, M. & Huang, H. Ribosome display: a potent display technology used for selecting and evolving specific binders with desired properties. Mol. Biotechnol. 61, 60–71 (2018).
Zhang, J. et al. Mechanisms of ribosome stalling by SecM at multiple elongation steps. eLife 4, e09684 (2015).
Tsai, A., Kornberg, G., Johansson, M., Chen, J. & Puglisi, J. D. The dynamics of SecM-induced translational stalling. Cell Rep. 7, 1521–1533 (2014).
Wei, J., Wu, C. & Sachs, M. S. The arginine attenuator peptide interferes with the ribosome peptidyl transferase center. Mol. Cell. Biol. 32, 2396–2406 (2012).
Sothiselvam, S. et al. Macrolide antibiotics allosterically predispose the ribosome for translation arrest. Proc. Natl Acad. Sci. USA 111, 9804–9809 (2014).
Kannan, K. et al. The general mode of translation inhibition by macrolide antibiotics. Proc. Natl Acad. Sci. USA 111, 15958–15963 (2014).
Dorner, S., Brunelle, J. L., Sharma, D. & Green, R. The hybrid state of tRNA binding is an authentic translation elongation intermediate. Nat. Struct. Mol. Biol. 13, 234–241 (2006).
Huter, P. et al. Structural basis for polyproline-mediated ribosome stalling and rescue by the translation elongation factor EF-P. Mol. Cell 68, 515–527.e6 (2017).
Alderete, J. P., Jarrahian, S. & Geballe, A. P. Translational effects of mutations and polymorphisms in a repressive upstream open reading frame of the human cytomegalovirus UL4 gene. J. Virol. 73, 8330–8337 (1999).
Brown, A., Shao, S., Murray, J., Hegde, R. S. & Ramakrishnan, V. Structural basis for stop codon recognition in eukaryotes. Nature 524, 493–496 (2015).
Joazeiro, C. A. P. Ribosomal stalling during translation: providing substrates for ribosome-associated protein quality control. Annu. Rev. Cell Dev. Biol. 33, 343–368 (2017).
Polikanov, Y. S., Steitz, T. A. & Innis, C. A. A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome. Nat. Struct. Mol. Biol. 21, 787–793 (2014).
Crooks, G. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Grant, T. & Grigorieff, N. Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6. eLife 4, e06980 (2015).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Bai, X.-C., Rajendra, E., Yang, G., Shi, Y. & Scheres, S. H. W. Sampling the conformational space of the catalytic subunit of human γ-secretase. eLife 4, e11182 (2015).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
van Heel, M. & Schatz, M. Fourier shell correlation threshold criteria. J. Struct. Biol. 151, 250–262 (2005).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Natchiar, S. K., Myasnikov, A. G., Kratzat, H., Hazemann, I. & Klaholz, B. P. Visualization of chemical modifications in the human 80S ribosome structure. Nature 551, 472–477 (2017).
Behrmann, E. et al. Structural snapshots of actively translating human ribosomes. Cell 161, 845–857 (2015).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of COOT. Acta Crystallogr. D. 66, 486–501 (2010).
Voorhees, R. M., Fernández, I. S., Scheres, S. H. W. & Hegde, R. S. Structure of the mammalian ribosome-Sec61 complex to 3.4 Å resolution. Cell 157, 1632–1643 (2014).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D. 68, 352–367 (2012).
Noeske, J. et al. High-resolution structure of the Escherichia coli ribosome. Nat. Struct. Mol. Biol. 22, 336–341 (2015).
Ufimtsev, I. S. & Martinez, T. J. Quantum chemistry on graphical processing units. 3. Analytical energy gradients, geometry optimization, and first principles molecular dynamics. J. Chem. Theory Comput. 5, 2619–2628 (2009).
Titov, A. V., Ufimtsev, I. S., Luehr, N. & Martinez, T. J. Generating efficient quantum chemistry codes for novel architectures. J. Chem. Theory Comput. 9, 213–221 (2013).
Smith, T., Heger, A. & Sudbery, I. UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 27, 491–499 (2017).
Shen, W., Le, S., Li, Y. & Hu, F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE 11, e0163962 (2016).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Acknowledgements
We thank D. Toso (Bay Area Cryo-EM consortium) and P. Tobias for help with microscope operation and data collection; V. Kasinath, J. Liu, K. Nguyen, B. Greber, F. Jiang and A. Myasnikov for discussions about EM data processing and N. Liaud, A. Lee, M. Tu, N. Lintner and N. Ingolia for helpful suggestions. This work was funded by the Pfizer Emerging Science Fund and by the NIH (grant no. R01-GM065050).
Author information
Authors and Affiliations
Contributions
K.F.M., S.L., R.G.D. and J.H.D.C. came up with the concept. W.L., F.R.W., K.F.M., S.T.-L.C., E.M. and J.H.D.C. conducted the investigation and formal analysis. W.L. and J.H.D.C. wrote the original draft of the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing interests. R.G.D. is employed by Pfizer, Inc. K.F.M. and S.L. were employed by Pfizer, Inc. The authors have filed a patent application related to this work.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Fig. 1 Analysis of affinity-purified RNCs.
a, In vitro translation assays of PCSK9 and CDH1 nascent chains with different affinity tags in the absence (white bar) or presence (grey bar) of 100 μM PF846 (data represent mean ± s.d., n = 3 independent experiments). Significance value: * p<0.1, *** p<0.001. b, Western blot with anti-FLAG antibody, of stalled PCSK9-RNCs purified using an N-terminal 3X-FLAG tag on the NC, and treated plus or minus RNase A after elution and pelleting through a sucrose cushion. Source data for panel a are available in Supplementary Data Set 3.
Supplementary Fig. 2 Cryo-EM data processing and model validation of PF846 stalled CDH1-RNCs.
a, The EM micrographs were first divided into 3 subsets for classification and refinement. The selected classes after refinement (labeled with red asterisks) were combined for an overall refinement. Signal subtraction using the A/P-tRNA, nascent chain and PF846 regions allowed classification and refinement of separate classes with A/A NC-tRNA or A/P NC-tRNA. The rest of the particles remaining after selecting the rotated state RNCs (labeled with blue asterisks) were merged and used for another refinement and 3D classification, which generated 4 different classes, including a non-rotated state with P/P NC-tRNA. Particle numbers at various steps of the reconstruction process are indicated. b, Final FSC curves of CDH1_RNCs, with the gold standard value of 0.143 used to define the resolution indicated. Particle numbers used for the final reconstructions are indicated. c, Model to map correlation with resolution at the FSC value of 0.5 shown.
Supplementary Fig. 3 Cryo-EM data processing of PF846 stalled PCSK9-RNCs.
a, Data processing steps for stalled PCSK9-RNCs, as described in Supplementary Fig. 2. Particle numbers at various steps of the reconstruction process are indicated. b, Final FSC curves of PCSK9-RNCs, with the “gold standard” value of 0.143 used to define the resolution indicated. Particle numbers used for the final reconstructions are indicated. c, Model to map correlations with resolution at FSC value of 0.5. d, Data processing steps for sample prepared with a short incubation time, which generated a higher ratio of non-rotated to rotated states of the ribosome. e, Final FSC curves of RNCs, with the “gold standard” value of 0.143 used to define the resolution as indicated.
Supplementary Fig. 4 Cryo-EM data processing and model validation of PF846 stalled USO1-RNCs.
a, Data processing steps for USO1-RNC complexes. Particle numbers at various steps of the reconstruction process are indicated. b, Final FSC curve of USO1-RNC, with the “gold standard” value of 0.143 used to define the resolution indicated. c, Model to map correlation with the resolution at an FSC value of 0.5.
Supplementary Fig. 5 Cryo-EM reconstructions of PF846-stalled PCSK9 and USO1 RNCs.
a, PCSK9-RNC in the rotated state with A/P and P/E tRNA, with 40S subunit (light blue), 60S subunit (grey), A/P site tRNA (green), P/E site tRNA (magenta) stalled PCSK9 nascent chain (blue) and PF846 (dark green) shown. b, PCSK9-RNC in the rotated state with A/A tRNA (orange) and P/E tRNA (magenta). c, Non-rotated state of the PCSK9-RNC stalled by PF846, with P/P tRNA (dark orange). d, USO1-RNC in the rotated state with A/P and P/E tRNA. Color coding as in panel (a), but with the NC in cyan.
Supplementary Fig. 6 Cryo-EM density maps for tRNA-mRNA base pairs in the CDH1-RNC complex in the rotated state with A/P and P/E tRNAs.
a, Density for A-site mRNA (orange) and anticodon stem-loop (ASL) region of A/P tRNA (green). Atoms shown other than carbon are colored blue (N), red (O), and orange (P). b, c, Models for an A-U, U-A, G-C or C-G base pairs between A/P tRNA anticodon nucleotide 34 (green) and mRNA codon nucleotide +6 (3rd nucleotide of codon in the A site)(orange). d, e, Models for A-U, U-A, G-C or C-G base pairs between A/P tRNA anticodon nucleotide 35 and mRNA codon nucleotide +5 (2nd nucleotide of codon in the A site). f, g, Models for A-U, U-A, G-C or C-G base pairs between A/P tRNA anticodon nucleotide 36 and mRNA codon nucleotide +4 (1st nucleotide of codon in the A site). h, Density for P-site mRNA (orange) and ASL of P/E tRNA (magenta). i, j, Models for an A-U, U-A, G-C or C-G base pairs between P/E tRNA anticodon nucleotide 34 (magenta) and mRNA codon nucleotide +3 (3rd nucleotide of codon in the P site)(orange). k, l, Models for A-U, U-A, G-C or C-G base pairs between P/E tRNA anticodon nucleotide 35 and mRNA codon nucleotide +2 (2nd nucleotide of codon in the P site). m, n, Models for A-U, U-A, G-C or C-G base pairs between P/E tRNA anticodon nucleotide 36 and mRNA codon nucleotide +1 (1st nucleotide of codon in the P site). All models are shown refined into the observed CDH1-RNC density. o, Observed density for representative A-U (A1052 and U1066) and G-C (G1065 and C1053) base pairs in 18S rRNA (cyan) in the 40S head and platform domains, near the P site.
Supplementary Fig. 7 Modeling of PF846 in stalled RNCs and the E. coli ribosome.
a, b, X-ray structures of PF846, with (a) as molecule 1 and (b) as molecule 210. c–f, Low energy conformations of PF846 based on quantum mechanical calculations starting from the X-ray structures of molecule 2. From c to f are poses 1 to 4 in Supplementary Table 1. Note that pose 4 was manually adjusted to fit the density by ~ 15° dihedral rotations of the chloropyridine and benzyl rings. g, Cartoon representation of the conserved small molecule binding loop from E. coli and human ribosomes, colored in dark cyan and gray, respectively. h, Residues that contribute to the binding of PF846 are shown in stick format (PDB: 4ybb). i–k, 23S rRNA residues from E. coli, which would have direct interactions with PF846 based on the corresponding positions in the human ribosome, and would lead to steric clashes. Dashed lines in c-e indicate the closest distances between the nucleotides and PF846. In E. coli, G745 is modified to N1-methyl-G, and U746 to ψ746, although the steric clashes would occur even with unmodified nucleotides.
Supplementary Fig. 8 Cryo-EM densities of PF846-stalled nascent chains.
Local resolution estimation for the PF846 stalled nascent chains from a, CDH1-RNC, b, PCSK9-RNC and c, USO1-RNC in the rotated state with A/P NC-tRNA, shown in the same orientation. The asterisks indicate the location of the peptidyl transferase center. d, e, Density map in the region where the (d) CDH1 (purple) and (e) PCSK9 (blue) nascent chains interact with the ribosome exit tunnel. Map density is shown in mesh, ribosomal proteins and rRNA nucleotides that have interaction with NC are shown in stick form (uL4 in green, uL22 in orange, eL39 in pink and 28S rRNA in cyan). The atomic models of the nascent chains are also shown in stick representation.
Supplementary Fig. 9 Asparagine scans of the CDH1 and PCSK9 NC sequences for effects on PF846-induced stalling.
a, Luciferase reporter assays using CDH1-derived NC sequences. CDH1 sequence is shown in full at the top, and locations of Asparagine (N) insertions are indicated. The approximate positioning of the stalled wild-type NC sequence relative to the PF846 binding site (PF) and PTC are marked with red and black arrows, respectively. Horizontal lines indicate the location of the PF and PTC libraries, for reference. Reactions were carried out in the absence (white bar) or presence (grey bar) of PF846 (data represent mean ± s.d., n = 3 independent experiments). b, Luciferase reporter assays using PCSK9-derived NC sequences. PCSK9 sequence is shown in full at the top, and locations of Asparagine (N) insertions are indicated. The approximate positioning of the stalled wild-type NC sequence relative to the PF846 binding site (PF) and PTC are marked with red and black arrows, respectively. Reactions were carried out in the absence (white bar) or presence (grey bar) of PF846 (data represent mean ± s.d., n = 3 independent experiments). Luciferase, Luciferase-only control sequence. Source data for panel a and b are available in Supplementary Data Set 3.
Supplementary Fig. 10 Selection of stalling sequences from mRNA libraries.
a, Schematic of mRNA library designs, with randomized locations in the CDH1 stalling sequence indicated. Numbers indicate amino acids in the CDH1 sequence. b, Protocol for mRNA library selections using in vitro translation reactions. RNase H treatment was carried out first during RNC binding and purification on anti-FLAG beads, and a second time after RNC release from the beads. c, Luciferase activity of translation reactions with luciferase reporters containing the CDH1 stalling sequence with the WT or RSCK motif near the PTC, as a function of PF846 concentration (data represent mean ± s.d., n = 3 independent experiments). Source data for panel c are available in Supplementary Data Set 3. d, Western blot of affinity-purified stalled CDH1-derived nascent chains. Reactions in the absence or presence of 50 µM PF846 are shown. Western blot of eRF1 for each sample is also shown, along with a Western of RPS19 as a loading control. e, MA plot of sequences enriched for PF846-induced translation elongation stalling, compared to translation reactions in the absence of PF846. Sequences were enriched from the mRNA library of CDH1-derived nascent chains with four amino acid positions randomized near the predicted location of PF846 binding in the ribosome exit tunnel. Log2-fold enrichment of sequences is plotted against total read count, for experiments carried out in duplicate. No sequences were enriched with an adjusted P-value < 0.01. Significance was tested by DESeq2 using the Wald test (two-sided) and adjusted for multiple comparisons by the Benjamini-Hochberg method39.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, W., Ward, F.R., McClure, K.F. et al. Structural basis for selective stalling of human ribosome nascent chain complexes by a drug-like molecule. Nat Struct Mol Biol 26, 501–509 (2019). https://doi.org/10.1038/s41594-019-0236-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-019-0236-8
This article is cited by
-
The eRF1 degrader SRI-41315 acts as a molecular glue at the ribosomal decoding center
Nature Chemical Biology (2024)
-
A molecular network of conserved factors keeps ribosomes dormant in the egg
Nature (2023)
-
Clinical, genetic and structural delineation of RPL13-related spondyloepimetaphyseal dysplasia suggest extra-ribosomal functions of eL13
npj Genomic Medicine (2023)
-
Regulation of the macrolide resistance ABC-F translation factor MsrD
Nature Communications (2023)
-
Signal peptide mimicry primes Sec61 for client-selective inhibition
Nature Chemical Biology (2023)