Abstract
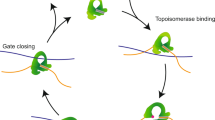
Type IA topoisomerases cleave single-stranded DNA and relieve negative supercoils in discrete steps corresponding to the passage of the intact DNA strand through the cleaved strand. Although type IA topoisomerases are assumed to accomplish this strand passage via a protein-mediated DNA gate, opening of this gate has never been observed. We developed a single-molecule assay to directly measure gate opening of the Escherichia coli type IA topoisomerases I and III. We found that after cleavage of single-stranded DNA, the protein gate opens by as much as 6.6 nm and can close against forces in excess of 16 pN. Key differences in the cleavage, ligation, and gate dynamics of these two enzymes provide insights into their different cellular functions. The single-molecule results are broadly consistent with conformational changes obtained from molecular dynamics simulations. These results allowed us to develop a mechanistic model of interactions between type IA topoisomerases and single-stranded DNA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data for Fig. 4 are available with the paper online. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Champoux, J. J. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 70, 369–413 (2001).
Corbett, K. D. & Berger, J. M. Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. Annu. Rev. Biophys. Biomol. Struct. 33, 95–118 (2004).
Liu, L. F., Liu, C.-C. & Alberts, B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell 19, 697–707 (1980).
Seol, Y., Zhang, H., Pommier, Y. & Neuman, K. C. A kinetic clutch governs religation by type IB topoisomerases and determines camptothecin sensitivity. Proc. Natl Acad. Sci. USA 109, 16125–16130 (2012).
Stewart, L., Redinbo, M. R., Qiu, X., Hol, W. G. & Champoux, J. J. A model for the mechanism of human topoisomerase I. Science 279, 1534–1541 (1998).
Li, Z., Mondragón, A. & DiGate, R. J. The mechanism of type IA topoisomerase-mediated DNA topological transformations. Mol. Cell 7, 301–307 (2001).
Dekker, N. H. et al. The mechanism of type IA topoisomerases. Proc. Natl Acad. Sci. USA 99, 12126–12131 (2002).
Tse-Dinh, Y. C. Biochemistry of bacterial type I DNA topoisomerases. Adv. Pharmacol. 29A, 21–37 (1994).
Srivenugopal, K. S., Lockshon, D. & Morris, D. R. Escherichia coli DNA topoisomerase III: purification and characterization of a new type I enzyme. Biochemistry 23, 1899–1906 (1984).
Confalonieri, F. et al. Reverse gyrase: a helicase-like domain and a type I topoisomerase in the same polypeptide. Proc. Natl Acad. Sci. USA 90, 4753–4757 (1993).
Goulaouic, H. et al. Purification and characterization of human DNA topoisomerase IIIalpha. Nucleic Acids Res. 27, 2443–2450 (1999).
Li, W. & Wang, J. C. Mammalian DNA topoisomerase IIIalpha is essential in early embryogenesis. Proc. Natl Acad. Sci. USA. 95, 1010–1013 (1998).
Seki, T., Seki, M., Onodera, R., Katada, T. & Enomoto, T. Cloning of cDNA encoding a novel mouse DNA topoisomerase III (Topo IIIbeta) possessing negatively supercoiled DNA relaxing activity, whose message is highly expressed in the testis. J. Biol. Chem. 273, 28553–28556 (1998).
Massé, E. & Drolet, M. Relaxation of transcription-induced negative supercoiling is an essential function of Escherichia coli DNA topoisomerase I. J. Biol. Chem. 274, 16654–16658 (1999).
Suski, C. & Marians, K. J. Resolution of converging replication forks by RecQ and topoisomerase III. Mol. Cell 30, 779–789 (2008).
Perez-Cheeks, B. A., Lee, C., Hayama, R. & Marians, K. J. A role for topoisomerase III in Escherichia coli chromosome segregation. Mol. Microbiol. 86, 1007–1022 (2012).
Cortés, F., Pastor, N., Mateos, S. & Domínguez, I. Roles of DNA topoisomerases in chromosome segregation and mitosis. Mutat. Res. 543, 59–66 (2003).
Goodwin, A., Wang, S.-W., Toda, T., Norbury, C. & Hickson, I. D. Topoisomerase III is essential for accurate nuclear division in Schizosaccharomyces pombe. Nucleic Acids Res. 27, 4050–4058 (1999).
Bocquet, N. et al. Structural and mechanistic insight into Holliday-junction dissolution by topoisomerase IIIα and RMI1. Nat. Struct. Mol. Biol. 21, 261–268 (2014).
Bussen, W., Raynard, S., Busygina, V., Singh, A. K. & Sung, P. Holliday junction processing activity of the BLM-Topo IIIalpha-BLAP75 complex. J. Biol. Chem. 282, 31484–31492 (2007).
Ahmad, M. et al. Topoisomerase 3β is the major topoisomerase for mRNAs and linked to neurodevelopment and mental dysfunction. Nucleic Acids Res. 45, 2704–2713 (2017).
Xu, D. et al. Top3β is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat. Neurosci. 16, 1238–1247 (2013).
Lima, C. D., Wang, J. C. & Mondragón, A. Three-dimensional structure of the 67K N-terminal fragment of E. coli DNA topoisomerase I. Nature 367, 138–146 (1994).
Mondragón, A. & DiGate, R. The structure of Escherichia coli DNA topoisomerase III. Structure 7, 1373–1383 (1999).
Feinberg, H., Lima, C. D. & Mondragón, A. Conformational changes in E. coli DNA topoisomerase I. Nat. Struct. Biol. 6, 918–922 (1999).
Baker, N. M., Rajan, R. & Mondragón, A. Structural studies of type I topoisomerases. Nucleic Acids Res. 37, 693–701 (2009).
Leelaram, M. N. et al. Type IA topoisomerase inhibition by clamp closure. FASEB J. 27, 3030–3038 (2013).
Xiong, B. et al. The type IA topoisomerase catalytic cycle: a normal mode analysis and molecular dynamics simulation. Proteins Struct. Funct. Bioinforma. 71, 1984–1994 (2008).
Gunn, K. H., Marko, J. F. & Mondragón, A. An orthogonal single-molecule experiment reveals multiple-attempt dynamics of type IA topoisomerases. Nat. Struct. Mol. Biol. 24, 484–490 (2017).
Seol, Y. & Neuman, K. Magnetic tweezers for single-molecule manipulation. in Single Molecule Analysis (eds. Peterman, E. J. G. & Wuite, G. J. L.) 265–293 (Humana Press, New York, 2011).
Mills, M. et al. RecQ helicase triggers a binding mode change in the SSB-DNA complex to efficiently initiate DNA unwinding. Nucleic Acids Res. 45, 11878–11890 (2017).
Perry, K. & Mondragón, A. Structure of a complex between E. coli DNA topoisomerase I and single-stranded DNA. Structure 11, 1349–1358 (2003).
Dekker, N. H. et al. Thermophilic topoisomerase I on a single DNA molecule. J. Mol. Biol. 329, 271–282 (2003).
Bell, G. I. Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978).
Bronson, J. E., Fei, J., Hofman, J. M., Gonzalez, R. L. Jr. & Wiggins, C. H. Learning rates and states from biophysical time series: a Bayesian approach to model selection and single-molecule FRET data. Biophys. J. 97, 3196–3205 (2009).
Terekhova, K., Gunn, K. H., Marko, J. F. & Mondragón, A. Bacterial topoisomerase I and topoisomerase III relax supercoiled DNA via distinct pathways. Nucleic Acids Res. 40, 10432–10440 (2012).
Nurse, P., Levine, C., Hassing, H. & Marians, K. J. Topoisomerase III can serve as the cellular decatenase in Escherichia coli. J. Biol. Chem. 278, 8653–8660 (2003).
Terekhova, K., Marko, J. F. & Mondragón, A. Single-molecule analysis uncovers the difference between the kinetics of DNA decatenation by bacterial topoisomerases I and III. Nucleic Acids Res. 42, 11657–11667 (2014).
Zhang, J., Pan, B., Li, Z., Sheng Zhao, X. & Huang, L. Kinetic insights into the temperature dependence of DNA strand cleavage and religation by topoisomerase III from the hyperthermophile Sulfolobus solfataricus. Sci. Rep. 7, 5494 (2017).
Domanico, P. L. & Tse-Dinh, Y. C. Mechanistic studies on E. coli DNA topoisomerase I: divalent ion effects. J. Inorg. Biochem. 42, 87–96 (1991).
Tse-Dinh, Y. C. Uncoupling of the DNA breaking and rejoining steps of Escherichia coli type I DNA topoisomerase: demonstration of an active covalent protein-DNA complex. J. Biol. Chem. 261, 10931–10935 (1986).
Sorokin, E. P. et al. Inhibition of Mg2+ binding and DNA religation by bacterial topoisomerase I via introduction of an additional positive charge into the active site region. Nucleic Acids Res. 36, 4788–4796 (2008).
Zhu, C. X., Roche, C. J. & Tse-Dinh, Y. C. Effect of Mg(II) binding on the structure and activity of Escherichia coli DNA topoisomerase I. J. Biol. Chem. 272, 16206–16210 (1997).
Bhat, A. G., Leelaram, M. N., Hegde, S. M. & Nagaraja, V. Deciphering the distinct role for the metal coordination motif in the catalytic activity of Mycobacterium smegmatis topoisomerase I. J. Mol. Biol. 393, 788–802 (2009).
Cao, N., Tan, K., Annamalai, T., Joachimiak, A. & Tse-Dinh, Y.-C. Investigating mycobacterial topoisomerase I mechanism from the analysis of metal and DNA substrate interactions at the active site. Nucleic Acids Res. 46, 7296–7308 (2018).
Torrie, G. M. & Valleau, J. P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: umbrella sampling. J. Comput. Phys. 23, 187–199 (1977).
Souaille, M. & Roux, B. Extension to the weighted histogram analysis method: combining umbrella sampling with free energy calculations. Comput. Phys. Commun. 135, 40–57 (2001).
Changela, A., DiGate, R. J. & Mondragón, A. Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule. Nature 411, 1077–1081 (2001).
Minh, D. D. L. Multidimensional potentials of mean force from biased experiments along a single coordinate. J. Phys. Chem. B 111, 4137–4140 (2007).
Li, Z., Mondragón, A., Hiasa, H., Marians, K. J. & DiGate, R. J. Identification of a unique domain essential for Escherichia coli DNA topoisomerase III-catalysed decatenation of replication intermediates. Mol. Microbiol. 35, 888–895 (2000).
Zechiedrich, E. L. et al. Roles of topoisomerases in maintaining steady-state DNA supercoiling in Escherichia coli. J. Biol. Chem. 275, 8103–8113 (2000).
Cejka, P., Plank, J. L., Dombrowski, C. C. & Kowalczykowski, S. C. Decatenation of DNA by the S. cerevisiae Sgs1-Top3-Rmi1 and RPA complex: a mechanism for disentangling chromosomes. Mol. Cell 47, 886–896 (2012).
Tse-Dinh, Y.-C. Targeting bacterial topoisomerase I to meet the challenge of finding new antibiotics. Future Med. Chem. 7, 459–471 (2015).
Sandhaus, S. et al. Small-molecule inhibitors targeting topoisomerase I as novel antituberculosis agents. Antimicrob. Agents Chemother. 60, 4028–4036 (2016).
Giles, G. I. & Sharma, R. P. Topoisomerase enzymes as therapeutic targets for cancer chemotherapy. Med. Chem. 1, 383–394 (2005).
Nagaraja, V., Godbole, A. A., Henderson, S. R. & Maxwell, A. DNA topoisomerase I and DNA gyrase as targets for TB therapy. Drug Discov. Today 22, 510–518 (2017).
Ahmed, W., Menon, S., Godbole, A. A., Karthik, P. V. D. N. B. & Nagaraja, V. Conditional silencing of topoisomerase I gene of Mycobacterium tuberculosis validates its essentiality for cell survival. FEMS Microbiol. Lett. 353, 116–123 (2014).
Ravishankar, S. et al. Genetic and chemical validation identifies Mycobacterium tuberculosis topoisomerase I as an attractive anti-tubercular target. Tuberculosis (Edinb) 95, 589–598 (2015).
Aedo, S. & Tse-Dinh, Y.-C. Isolation and quantitation of topoisomerase complexes accumulated on Escherichia coli chromosomal DNA. Antimicrob. Agents Chemother. 56, 5458–5464 (2012).
Pommier, Y. & Marchand, C. Interfacial inhibitors: targeting macromolecular complexes. Nat. Rev. Drug. Discov. 11, 25–36 (2011).
Seol, Y., Hardin, A. H., Strub, M.-P., Charvin, G. & Neuman, K. C. Comparison of DNA decatenation by Escherichia coli topoisomerase IV and topoisomerase III: implications for non-equilibrium topology simplification. Nucleic Acids Res. 41, 4640–4649 (2013).
Zhu, C.-X. & Tse-Dinh, Y.-C. Overexpression and purification of bacterial DNA topoisomerase I. in DNA Topoisomerase Protocols 145–151 (Humana Press, New York, 1999).
Seol, Y., Strub, M.-P. & Neuman, K. C. Single molecule measurements of DNA helicase activity with magnetic tweezers and t-test based step-finding analysis. Methods 105, 119–127 (2016).
Kalé, L. et al. NAMD2: greater scalability for parallel molecular dynamics. J. Comput. Phys. 151, 283–312 (1999).
Brooks, B. R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
Huang, J. & MacKerell, A. D. Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Neria, E., Fischer, S. & Karplus, M. Simulation of activation free energies in molecular systems. J. Chem. Phys. 105, 1902–1921 (1996).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 27–28 (1996).
Acknowledgements
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health (HL001056–07 to K.C.N.) and a grant from the National Institutes of Health (R01GM054226 to Y.-C.T.-D.). This work used the computational resources of the NIH HPC Biowulf cluster. We thank Y. Seol (National Heart, Lung, and Blood Institute, National Institutes of Health) for insightful discussions, for experimental assistance, and for providing the DNA substrates. We thank L. Bradley (National Heart, Lung, and Blood Institute, National Institutes of Health) for assistance with purifying topoisomerase III and B. Cheng (New York Medical College) for purified topoisomerase I.
Author information
Authors and Affiliations
Contributions
M.M. and K.C.N. conceived the experiments. M.M. conducted the experiments and simulations and analyzed the data. Y.-C.T.-D. provided materials. M.M., K.C.N., and Y.-C.T.-D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Hairpin-gate-opening analysis.
(a) Comparison of DNA extension of an individual tether before (left) and after (right) the addition of 500 pM topo I. Black lines are DNA extension, blue lines are applied force (9–24 pN). Extension increases are above the baseline extension of the tether in the absence of protein. (b) Histograms of DNA extension of the same tether under force before and after addition of protein. (c-d) Examples of multi-Gaussian fits to a single unfolding cycle for topo III (c) and topo I (d). Histograms of smoothed data are in red, Residuals are shown in the top graph, individual Gaussians in the bottom graph. The overall fit (blue line) is shown overlaid on the histograms in the center graph. Inset in (c) shows an example of smoothed data for two events (black lines). Raw data is shown in gray. (e-f) Histograms of gate opening extension increases for topo III (ntethers = 4, ncycles = 58) (e) and topo I (ntethers = 5, ncycles = 25) (f). Error bars are s.e.m. Black lines are Gaussian fits. (g) Average survival times for tethers in the presence of 1 % SDS. Note that the time scale is logarithmic. Data was combined for topo I (ntethers = 3) and topo III (ntethers = 2). Average lifetime of tethers with evidence of cleavage was 27.7 ± 5.7 s. Average lifetime of the DNA tethers alone in 1 % SDS was 1486 ± 663 s. Error bars are s.d
Supplementary Figure 2 Extension-change distributions of the gapped DNA substrate.
Distance distributions (red) for the DNA extension change measured with the gapped DNA substrate for topo III at 8, 10, 12, 14 and 16 pN (ntethers = 8) and topo I at 12, 14, 16, 18 pN (ntethers = 7). Bin size is 0.5 nm. Blue lines are Gaussian fits obtained using the Igor multipeak fitting algorithm. Error bars are s.e.m. KD and extension change values are reported in Supplementary Table 1
Supplementary Figure 3 Closed-state lifetimes.
(a) Histograms of topo III short-lived closed state lifetimes (t ≤ 20 s), bin size 0.2–0.5 s, ntethers = 8. Data were fit to single exponentials (black) to determine kopen. Reduced χ2 values from exponential fits are 0.76 (8 pN), 1 (10 pN), 0.74 (12 pN), 1.2 (14 pN), and 1.1 (16 pN). (b) Histograms of topo III long-lived closed state lifetimes (t > 20 s), bin size 10–50 s, ntethers = 8. Data were fit to single exponentials (black) to determine kcleavage. Reduced χ2 values from exponential fits are 0.58 (8 pN), 0.5 (10 pN), 0.11 (12 pN), 0.36 (14 pN), and 0.76 (16 pN). (c) Histograms of topo I closed state lifetimes (bin size 0.1–0.2 s), ntethers = 7. Black lines are double-exponential fits. Reduced χ2 values are 1.1 (12 pN), 1.1 (14 pN), 0.56 (16 pN), and 0.72 (18 pN). Gray lines are single exponential fits. Reduced χ2 values are 1.6 (12 pN), 2.4 (14 pN), 1.1 for (16 pN), and 1.5 (18 pN). Comparison of these fits using an F-test resulted in F values of 8.9 for (12 pN), p = 0.00093, 22.2 (14 pN), p < 0.00001, 13.6 (16 pN), p = 0.00011, and 30.0 (18 pN), p < 0.00001. The faster rate was assumed to be kopen and the slower rate to be 1/(tcleavage + topen). Rates obtained from the fits are shown in Supplementary Table 2. Error bars are s.e.m
Supplementary Figure 4 Open-state-lifetime distributions.
(a) Histograms of topo III open state lifetimes, bin size 2–5 s, ntethers = 8. Exponential fits in black. Reduced χ2 values are 0.22 (8 pN), 1.1 (10 pN), 1.5 (12 pN), 0.56 (14 pN), and 1.3 (16 pN). (b) Histograms of topo I open state, bin size 0.2–0.5 s, ntethers = 7. Exponential fits are shown in black. Reduced χ2 values are 2.7 (12 pN), 1.5 (14 pN), 2.7 (16 pN), and 1.9 (18 pN). Rates from the fits are shown in Supplementary Table 2. Error bars are s.e.m
Supplementary Figure 5 Effects of magnesium on topo-DNA binding.
(a) Average number of binding events (solid bars) and gate opening events (striped bars) from hairpin opening and refolding experiments in the presence (topo III ntethers = 4, ncycles = 58, topo I ntethers = 5, ncycles = 25) and absence of added magnesium. Error bars are s.d. In the absence of magnesium (topo III ntethers = 2, ncycles = 18, topo I ntethers = 4, ncycles = 8), less than half the number of bound proteins was observed for both topo III and topo I, whereas the ratio of bound proteins to opening events remained constant. For 0 Mg2+ results, only tethers for which at least one binding event was observed were counted, resulting in a possible over-estimation of the average number of binding events. (b) Example extension as a function of time at 14 pN for topo I at 3 mM Mg2+ and 0 mM Mg2+ with EDTA. Religation is reduced but not eliminated even under chelating conditions
Supplementary Figure 6 Details of free-energy calculations from simulations.
(a-b) Force dependent free energies. The equilibrium free energy calculated from the simulations is shown in black, along with ΔGF curves for F = 8 pN (dark gray), F = 12 pN (medium gray), F = 16 pN (light gray). (a) Force-dependent free energy profile in which the entire free energy profile is force dependent. (b) Force-dependent free energy in which the initial opening depends on a force-independent conformational change. (c) Heat map of a two-dimensional free energy profile along the restrained umbrella sampling reaction coordinate, the distance between the centers of mass of domains III and IV, and x, the distance between the catalytic tyrosine and the DNA cleavage site. Two parallel pathways can be observed, one in which the decatenation loop forms contacts with an acidic loop in domain II, and one in which the decatenation loop makes no contacts with the rest of the protein. (d) Structures of open state without decatenation latch. In the absence of latch formation, the hinge region of domain II adopts a more extended conformation. (e) Open structure of topo III from simulations (cyan) aligned with the structure of an isolated fragment of topo I consisting of domains II and III (purple, PDBID: 1CYY, chain A). The predicted structure of domain II in the open state based on the fragment conformation is similar to the simulation structure. (f) Structural alignment rotated to show differences in curvature of the β sheet
Supplementary Figure 7 Negative-supercoil relaxation by topo I and topo III at high force.
(a) Schematic of high force relaxation experiments (not to scale). DNA was negatively supercoiled at low force (F < 0.5 pN). The force was then raised to 12 pN for 5 to 15 min, after which is was lowered again. Relaxation was observed as a change in the linking number inferred from the change in extension after decreasing the force. The low force extension was compared with the original low force extension as a function of linking number curve measured in (a). (b) Examples of high force relaxation events for topo III and topo I. Blue lines represent applied force, black lines are DNA extension. Green arrows indicate rotation of magnets. Before and after hat curves are also shown. The fact that the molecules could be supercoiled after relaxation demonstrates that they were relaxed by the topoisomerase and not nicked, whereas the shift in the center of the extension versus magnet rotation curves verifies that the negative supercoiling was relaxed by the topo IA enzyme. Both proteins required more time to relax DNA at 12 pN than at forces below 0.5 pN
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Notes 1 and 2, and Supplementary Tables 1–4
Supplementary Video 1
Gate dynamics from MD simulations. Changes in the topo III structure over the course of the umbrella sampling simulations. Domain I is shown in red and domain IV in blue. The black dashed line indicates the distance between the catalytic tyrosine (green) and the cleavage site on the DNA backbone
Supplementary Video 2
Interactions between decatenation loop and acidic loop from simulations. The decatenation loop is shown in blue and the acidic loop in red. Key residues are indicated
Source data
Rights and permissions
About this article
Cite this article
Mills, M., Tse-Dinh, YC. & Neuman, K.C. Direct observation of topoisomerase IA gate dynamics. Nat Struct Mol Biol 25, 1111–1118 (2018). https://doi.org/10.1038/s41594-018-0158-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-018-0158-x
This article is cited by
-
Unraveling topoisomerase IA gate dynamics in presence of PPEF and its preclinical evaluation against multidrug-resistant pathogens
Communications Biology (2023)
-
Extracting time series matching a small-angle X-ray scattering profile from trajectories of molecular dynamics simulations
Scientific Reports (2022)
-
Structural and biochemical basis for DNA and RNA catalysis by human Topoisomerase 3β
Nature Communications (2022)
-
Duplex DNA and BLM regulate gate opening by the human TopoIIIα-RMI1-RMI2 complex
Nature Communications (2022)