Abstract
The cell division cycle consists of a series of temporally ordered events. Cell cycle kinases and phosphatases provide key regulatory input, but how the correct substrate phosphorylation and dephosphorylation timing is achieved is incompletely understood. Here we identify a PxL substrate recognition motif that instructs dephosphorylation by the budding yeast Cdc14 phosphatase during mitotic exit. The PxL motif was prevalent in Cdc14-binding peptides enriched in a phage display screen of native disordered protein regions. PxL motif removal from the Cdc14 substrate Cbk1 delays its dephosphorylation, whereas addition of the motif advances dephosphorylation of otherwise late Cdc14 substrates. Crystal structures of Cdc14 bound to three PxL motif substrate peptides provide a molecular explanation for PxL motif recognition on the phosphatase surface. Our results illustrate the sophistication of phosphatase–substrate interactions and identify them as an important determinant of ordered cell cycle progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Atomic coordinates and structure factors have been deposited in the Protein Data Bank, with accession numbers 6G84 (Cdc14:Cbk1-C2), 6G85 (Cdc14:Cbk1-P212121) and 6G86 (Cdc14:Sic1). All additional data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Morgan, D. The Cell Cycle: Principles of Control (New Science Press, London, 2007).
Loog, M. & Morgan, D. O. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434, 104–108 (2005).
Koivomägi, M. et al. Dynamics of Cdk1 substrate specificity during the cell cycle. Mol. Cell 42, 610–623 (2011).
Bhaduri, S. & Pryciak, P. M. Cyclin-specific docking motifs promote phosphorylation of yeast signaling proteins by G1/S Cdk complexes. Curr. Biol. 21, 1615–1623 (2011).
Godfrey, M. et al. PP2ACdc55 phosphatase imposes ordered cell-cycle phosphorylation by opposing threonine phosphorylation. Mol. Cell 65, 393–402 (2017).
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709–718 (1998).
Guacci, V., Hogan, E. & Koshland, D. Chromosome condensation and sister chromatid pairing in budding yeast. J. Cell Biol. 125, 517–530 (1994).
Higuchi, T. & Uhlmann, F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature 433, 171–176 (2005).
Woodbury, E. L. & Morgan, D. O. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat. Cell Biol. 9, 106–112 (2007).
Khmelinskii, A., Roostalu, J., Roque, H., Antony, C. & Schiebel, E. Phosphorylation-dependent protein interactions at the spindle midzone mediate cell cycle regulation of spindle elongation. Dev. Cell 17, 244–256 (2009).
Mirchenko, L. & Uhlmann, F. Sli15INCENP dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset. Curr. Biol. 20, 1396–1401 (2010).
Kuilman, T. et al. Identification of Cdk targets that control cytokinesis. EMBO J. 34, 81–96 (2014).
Bloom, J. et al. Global analysis of Cdc14 phosphatase reveals diverse roles in mitotic processes. J. Biol. Chem. 286, 5434–5445 (2011).
Touati, S. A., Kataria, M., Jones, A. W., Snijders, A. P. & Uhlmann, F. Phosphoproteome dynamics during mitotic exit in budding yeast. EMBO J. 37, e98745 (2018).
Visintin, R., Hwang, E. S. & Amon, A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398, 818–823 (1999).
Shou, W. et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97, 233–244 (1999).
Bouchoux, C. & Uhlmann, F. A quantitative model for ordered Cdk substrate dephosphorylation during mitotic exit. Cell 147, 803–814 (2011).
Breitkreutz, A. et al. A global protein kinase and phosphatase interaction network in yeast. Science 328, 1043–1046 (2010).
Kao, L. et al. Global analysis of Cdc14 dephosphorylation sites reveals essential regulatory role in mitosis and cytokinesis. Mol. Cell Proteom. 13, 594–605 (2014).
Suzuki, K. et al. Identification of non-Ser/Thr-Pro consensus motifs for Cdk1 and their roles in mitotic regulation of C2H2 zinc finger proteins and Ect2. Sci. Rep. 5, 7929 (2015).
Gray, C. H., Good, V. M., Tonks, N. K. & Barford, D. The structure of the cell cycle protein Cdc14 reveals a proline-directed protein phosphatase. EMBO J. 22, 3524–3535 (2003).
Bremmer, S. C. et al. Cdc14 phosphatases preferentially dephosphorylate a subset of cyclin-dependent kinase (Cdk) sites containing phosphoserine. J. Biol. Chem. 287, 1662–1669 (2012).
Eissler, C. L. et al. The Cdk/Cdc14 module controls activation of the Yen1 Holliday junction resolvase to promote genome stability. Mol. Cell 54, 80–93 (2014).
Seo, M. H., Nim, S., Jeon, J. & Kim, P. M. Large-scale interaction profiling of protein domains through proteomic peptide-phage display using custom peptidomes. Methods Mol. Biol. 1518, 213–226 (2017).
Davey, N. E. et al. Attributes of short linear motifs. Mol. Biosyst. 8, 268–281 (2012).
Colaert, N., Helsens, K., Martens, L., Vandekerckhove, J. & Gevaert, K. Improved visualization of protein consensus sequences by iceLogo. Nat. Methods 6, 786–787 (2009).
Brace, J., Hsu, J. & Weiss, E. L. Mitotic exit control of the Saccharomyces cerevisiae Ndr/LATS kinase Cbk1 regulates daughter cell separation after cytokinesis. Mol. Cell. Biol. 31, 721–735 (2011).
Wienken, C. J., Baaske, P., Rothbauer, U., Braun, D. & Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1, 100 (2010).
Ivarsson, Y. et al. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc. Natl Acad. Sci. USA 111, 2542–2547 (2014).
Colman-Lerner, A., Chin, T. E. & Brent, R. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107, 739–750 (2001).
Korinek, W. S. et al. Cyk3, a novel SH3-domain protein, affects cytokinesis in yeast. Curr. Biol. 10, 947–950 (2000).
Meitinger, F. et al. Targeted localization of Inn1, Cyk3 and Chs2 by the mitotic-exit network regulates cytokinesis in budding yeast. J. Cell Sci. 123, 1851–1861 (2010).
Taylor, G. S., Liu, Y., Baskerville, C. & Charbonneau, H. The activity of Cdc14p, an oligomeric dual specificity protein phosphatase from Saccharomyces cerevisiae, is required for cell cycle progression. J. Biol. Chem. 272, 24054–24063 (1997).
Kobayashi, J. & Matsuura, Y. Structure and dimerization of the catalytic domain of the protein phosphatase Cdc14p, a key regulator of mitotic exit in Saccharomyces cerevisiae. Protein Sci. 26, 2105–2112 (2017).
Wood, J. S. & Hartwell, L. H. A dependent pathway of gene functions leading to chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 94, 718–726 (1982).
Nguyen, V. Q., Co, C. & Li, J. J. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature 411, 1068–1073 (2001).
Traverso, E. E. et al. Characterization of the Net1 cell cycle-dependent regulator of the Cdc14 phosphatase from budding yeast. J. Biol. Chem. 276, 21924–21931 (2001).
Shou, W. & Deshaies, R. J. Multiple telophase arrest bypassed (tab) mutants alleviate the essential requirement for Cdc15 in exit from mitosis in S. cerevisiae. BMC Genetics 3, 4 (2002).
Azzam, R. et al. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 305, 516–519 (2004).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Bollen, M., Peti, W., Ragusa, M. J. & Beullens, M. The extended PP1 toolkit: designed to create specificity. Trends Biochem. Sci. 35, 450–458 (2010).
Hertz, E. P. T. et al. A conserved motif provides binding specificity for the PP2A-B56 phosphatase. Mol. Cell 63, 686–695 (2016).
Swanley, D. L. et al. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 10, 676–682 (2013).
Holt, L. J. et al. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682–1686 (2009).
López-Avilés, S., Kapuy, O., Novák, B. & Uhlmann, F. Irreversibility of mitotic exit is the consequence of systems-level feedback. Nature 459, 592–595 (2009).
Davey, N. E. et al. Discovery of short linear motif-mediated interactions through phage display of intrinsically disordered regions of the human proteome. FEBS J. 284, 485–498 (2017).
Sun, M. G., Seo, M. H., Nim, S., Corbi-Verge, C. & Kim, P. M. Protein engineering by highly parallel screening of computationally designed variants. Sci. Adv. 2, e1600692 (2016).
Frank, R. High-density synthetic peptide microarrays: emerging tools for functional genomics and proteomics. Comb. Chem. High Throughput Screen. 5, 429–440 (2002).
Winter, G., Lobley, C. M. & Prince, S. M. Decision making in xia2. Acta Crystallogr. 69, 1260–1273 (2013).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. 66, 486–501 (2010).
Vaguine, A. A., Richelle, J. & Wodak, S. J. SFCHECK: a unified set of procedures for evaluating the quality of macromolecular structure-factor data and their agreement with the atomic model. Acta Crystallogr. 55, 191–205 (1999).
Knop, M. et al. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15, 963–972 (1999).
O’Reilly, N., Charbin, A., Lopez-Serra, L. & Uhlmann, F. Facile synthesis of budding yeast α-factor and its use to synchronize cells of α mating type. Yeast 29, 233–240 (2012).
Uhlmann, F., Lottspeich, F. & Nasmyth, K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37–42 (1999).
Foiani, M., Marini, F., Gamba, D., Lucchini, G. & Plevani, P. The B subunit of the DNA polymerase α-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol. Cell. Biol. 14, 923–933 (1994).
Donovan, S., Harwood, J., Drury, L. S. & Diffley, J. F. Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc. Natl Acad. Sci. USA 94, 5611–5616 (1997).
Chatr-Aryamontri, A. et al. The BioGRID interaction database: 2017 update. Nucleic Acids Res. 45, D369–D379 (2017).
Acknowledgements
We thank N. Davey and D. Morgan for bringing together the Kim and Uhlmann laboratories; the Diamond Light Source Synchrotron for access to beamlines IO3, IO4 and IO4-1 (mx13775); S. Federico, D. Joshi and N. O’Reilly, The Francis Crick Institute, London, UK for peptide synthesis; J. Diffley, The Francis Crick Institute, London, UK for the antibody to Orc2; V. Christodoulou, M. Hall, S. Kjaer, L. Masino, A. Purkiss and S. Touati for help and advice; and M. Godfrey, E. Weiss and members of our laboratory for discussions and critical reading of the manuscript. This work was supported by The Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001198), the UK Medical Research Council (FC001198) and the Wellcome Trust (FC001198).
Author information
Authors and Affiliations
Contributions
M.K. and F.U. conceived the study; M.K. performed the experiments; S.M. and M.K. solved the crystal structures; M.-H.S., C.C.-V. and P.M.K. conducted the phage display screen; and M.K. and F.U. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Biochemical characterization of the PxL motif.
(a), Recombinant Cdc14 preparations used for the phage display screen (His-Cdc14), peptide array (HA-Cdc14) and microscale thermophoresis experiments (GFP-Cdc14) were analyzed by SDS–PAGE and stained with Coomassie blue. A size marker is included. (b), Peptides recovered in the phage display screen are enriched in known Cdc14 substrates and Cdc14 interactors12,19 (hypergeometric test). (c), Mutational Cbk1 peptide array to probe the contribution of individual positions to Cdc14 binding. As in Fig. 1c, but a different membrane solvation was used (bottom), as indicated. A picture of the peptides stained with Ponceau S is also shown (top), as well as a control using the anti-HA antibody only (middle). (d), Microscale thermophoresis profiles of additional variant Cbk1-derived PxL motif peptides binding to GFP-Cdc14. Shown are the means and s.d. from two (F82G, V85G and Δ91–97) or three (L88G) independent experiments.
Supplementary Figure 2 Crystallization of Cdc14 with Cbk1 and Sic1 peptides.
(a), Schematic representation of the Cdc141–374 purification scheme, used for the crystallization studies. Purified protein was analyzed by SDS–PAGE followed by Coomassie blue staining. (b), Peptide docking to Cdc14 enhances its thermal stability. Thermal shift assays to determine the Cdc14 melting point (Tm) in the absence or presence of 200 μM Cbk1-derived PxL motif peptide or a scrambled derivative. Means and s.d. from three repeats of the experiment are shown and the melting points are listed together with the coefficient of determination (R2) that indicates the goodness of a fit to a Boltzmann sigmoidal function. (c), Photos of crystals obtained in the indicated space groups of Cdc14 bound by the Cbk1 and Sic1-derived PxL motif peptides.
Supplementary Figure 3 Comparison of budding yeast Cdc14 with human Cdc14B.
(a), Overlay of human Cdc14B (PDB ID: 1OHE; blue) and an S. cerevisiae Cdc14 protomer (gray), bound by the Cbk1 peptide (pink), demonstrates their similar overall fold (root-mean-square deviation of 0.89 Å over 242 Cα atoms). (b), Structure of the Cdc14-Cbk1 peptide complex. Each Cdc14 protomer (shades of gray) binds a Cbk1 peptide (pink). Close-up views of the MES buffer molecule bound to an active site and a zinc binding site are shown. (c), Anomalous difference electron density map indicating a density peak corresponding to zinc. The electron density is contoured at 7σ. For details regarding data collection, see Table 1. The zinc binding sites of both Cdc14 protomers are shown.
Supplementary Figure 4 Structures of the PxL motif peptides.
The difference electron density maps calculated by omitting the PxL peptides, contoured at 3σ, are displayed for Cbk1 and Sic1-derived PxL motif peptides bound to each Cdc14 protomer in the three crystal structures.
Supplementary Figure 5 Overlay of Cbk1 and Sic1-derived PxL motif peptides.
(a), Overlay of the Cbk1-derived PxL motif peptides bound to the hydrophobic pockets of the two Cdc14 protomers in the orthorhombic crystal. (b), Overlay of the Cbk1-derived PxL motif peptides bound to the hydrophobic pockets of the two Cdc14 protomers in the monoclinic crystal. (c), Overlay of the Sic1-derived PxL motif peptides bound to the hydrophobic pockets of the two Cdc14 protomers. (d), Overlay of the Cbk1 and the Sic1-derived PxL motif peptides bound to one of the hydrophobic Cdc14 pockets in the two orthorhombic crystals.
Supplementary Figure 6 Characterization of the Cdc14 Q106L and Cdc14 W108R mutant proteins.
(a), Cdc14 Q106L and Cdc14 W108R show uncompromised enzymatic activity. GFP-Cdc14, GFP-Cdc14 Q106L and GFP-Cdc14 W108R were purified and analyzed by SDS–PAGE followed by Coomassie blue staining. Velocity of p-NPP hydrolysis by GFP-Cdc14, GFP-Cdc14 Q106L and GFP-Cdc14 W108R was recorded as a function of the indicated substrate concentrations in three independent experiments. The means are shown; error bars indicate s.d. (b), GFP-Cdc14 Q106L and GFP-Cdc14 W108R fail to bind the Cbk1-derived PxL motif peptide. The means and s.d. from three independent microscale thermophoresis experiments to measure Cbk1-derived PxL motif peptide binding to the two Cdc14 variants are shown.
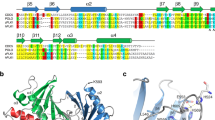
Supplementary Figure 7 Conservation of structurally identified Cdc14 features in its orthologs.
Multiple-sequence alignment of Cdc14 paralogs in different species. Secondary structure elements within the S. cerevisiae protein are indicated. Cdc14 residues, forming the PxL motif binding pocket and the dimer interface, are highlighted with blue and green stars, respectively. The proline residues found at the focal point of the dimer interface are highlighted with a green background. The residues coordinating zinc in S. cerevisiae Cdc14 are indicated by red stars; the catalytic cysteine residue in the active site is indicated by a gold star.
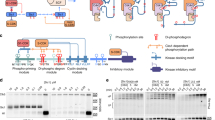
Supplementary Figure 8 The Cdc14 GETS dimer interface mutant.
(a), Additional illustration of the dimer interface within Cdc14, spanning both domains A and B and comprising extensive interactions between the two protomers. Residues at the dimer interface are displayed as sticks; a network of hydrogen bonds between the protomers is indicated. (b), Equal amounts of purified recombinant His6-Cdc14 and of the dimer interface mutant His6-Cdc14-GETS were analyzed by SDS–PAGE followed by Coomassie blue staining. (c), Analytical gel filtration profiles of Cdc14 and Cdc14-GETS using a Superdex 200 10/300 Increase column. The increased elution volume of Cdc14-GETS is suggestive of dimer disruption. (d), Velocity of p-NPP hydrolysis by 100 nM Cdc14 and Cdc14-GETS at the indicated substrate concentrations suggests that dimerization is required for full enzymatic activity of the phosphatase. The means and s.d. of three independent experiments are shown.
Supplementary Figure 9 Mechanism of PxL-motif-promoted dephosphorylation.
(a), Depiction of the distances from the C-terminal end of the resolved PxL motif peptide to the MES molecule found in the phosphatase active site in the same (cis) or adjacent (trans) protomer. (b), No evidence for allosteric phosphatase activation by the PxL motif. Velocity of p-NPP hydrolysis by Cdc14 was recorded in the presence of the indicated concentrations of wild-type or scrambled Cbk1-derived PxL motif peptide. The means and s.d. from three independent experiments are shown. (c), Kinetics analysis of peptide dephosphorylation by Cdc14 using an optimal phospho-SPxKK substrate containing an upstream functional or mutant PxL motif. (d), A PxL motif facilitates threonine dephosphorylation by Cdc14. Dephosphorylation velocities were determined using 1 μM Cdc14 and the indicated phosphopeptide concentrations, containing a pTP site preceded by a functional or mutant PxL motif.
Supplementary Figure 10 Distribution of PxL motifs relative to Cdk phosphorylation sites.
Relative positions of PxL motifs and known Cdk phosphorylation sites (based on PhosphoGRID, https://phosphogrid.org/, and the Saccharomyces Genome Database, https://www.yeastgenome.org/) on a representation of the ten top PxL-motif-containing phage display hits, together with a representation of the Orc6 PxL fusion protein.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 2–4
Supplementary Table 1
Peptides recovered in the phage display screen
Supplementary Dataset 1
Uncropped blot images
Rights and permissions
About this article
Cite this article
Kataria, M., Mouilleron, S., Seo, MH. et al. A PxL motif promotes timely cell cycle substrate dephosphorylation by the Cdc14 phosphatase. Nat Struct Mol Biol 25, 1093–1102 (2018). https://doi.org/10.1038/s41594-018-0152-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-018-0152-3