Abstract
Little is known about the intermolecular dynamics and stoichiometry of the interactions of the human immunodeficiency virus type 1 (HIV-1) envelope (Env) protein with its receptors and co-receptors on the host cell surface. Here we analyze time-resolved HIV-1 Env interactions with T-cell surface glycoprotein CD4 (CD4) and C-C chemokine receptor type 5 (CCR5) or C-X-C chemokine receptor type 4 (CXCR4) on the surface of cells, by combining multicolor super-resolution localization microscopy (direct stochastic optical reconstruction microscopy) with fluorescence fluctuation spectroscopy imaging. Utilizing the primary isolate JR-FL and laboratory HXB2 strains, we reveal the time-resolved stoichiometry of CD4 and CCR5 or CXCR4 in the pre-fusion complex with HIV-1 Env. The HIV-1 Env pre-fusion dynamics for both R5- and X4-tropic strains consists of a three-step mechanism, which seems to differ in stoichiometry. Analyses with the monoclonal HIV-1-neutralizing antibody b12 indicate that the mechanism of inhibition differs between JR-FL and HXB2 Env. The molecular insights obtained here identify assemblies of HIV-1 Env with receptors and co-receptors as potential novel targets for inhibitor design.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
09 May 2019
In the version of this article initially published, the label above the top right plot in Fig. 3b (HXB2-Alexa Fluor 488) was incorrect. The correct label is ‘HXB2-Alexa Fluor 405’. The error has been corrected in the HTML and PDF versions of the article.
References
Jakobsdottir, G. M. et al. On the whereabouts of HIV-1 cellular entry and its fusion ports. Trends Mol. Med. 23, 932–944 (2017).
Ozorowski, G. et al. Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Nature 547, 360–363 (2017).
Brandenberg, O. F., Magnus, C., Regoes, R. R. & Trkola, A. The HIV-1 entry process: a stoichiometric view. Trends Microbiol. 23, 763–774 (2015).
Digman, M. A., Dalal, R., Horwitz, A. F. & Gratton, E. Mapping the number of molecules and brightness in the laser scanning microscope. Biophys. J. 94, 2320–2332 (2008).
Digman, M. A., Wiseman, P. W., Choi, C., Horwitz, A. R. & Gratton, E. Stoichiometry of molecular complexes at adhesions in living cells. Proc. Natl Acad. Sci. USA. 106, 2170–2175 (2009).
Nolan, R. et al. nandb-number and brightness in R with a novel automatic detrending algorithm. Bioinformatics 33, 3508–3510 (2017).
Padilla-Parra, S., Marin, M., Kondo, N. & Melikyan, G. B. Synchronized retrovirus fusion in cells expressing alternative receptor isoforms releases the viral core into distinct sub-cellular compartments. PLoS Pathog. 8, e1002694 (2012).
Jones, D. M. & Padilla-Parra, S. Imaging real-time HIV-1 virion fusion with FRET-based biosensors. Sci. Rep. 5, 13449 (2015).
Healey, E. G. et al. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat. Struct. Mol. Biol. 22, 458–465 (2015).
Ratner, L. et al. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res. Hum. Retroviruses 3, 57–69 (1987).
Schülke, N. et al. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1gp140 envelope glycoprotein. J. Virol. 76, 7760–7776 (2002).
Francis, A. C. & Melikyan, G. B. Single HIV-1 imaging reveals progression of infection through CA-dependent steps of docking at the nuclear pore, uncoating, and nuclear transport. Cell Host Microbe 23, 536–548.e6 (2018).
Zaitseva, E. et al. Fusion stage of HIV-1 entry depends on virus-induced cell surface exposure of phosphatidylserine. Cell Host Microbe 22, 99–110.e7 (2017).
Jones, D. M. et al. Dynamin-2 stabilizes the HIV-1 fusion pore with a low oligomeric state. Cell Rep. 18, 443–453 (2017).
Russell, R. A. et al. Astrocytes resist HIV-1 fusion but engulf infected macrophage material. Cell Rep. 18, 1473–1483 (2017).
Padilla-Parra, S. et al. Fusion of mature HIV-1 particles leads to complete release of a gag-GFP-based content marker and raises the intraviral pH. PLoS One 8, e71002 (2013).
Kapanidis, A. N. et al. Alternating-laser excitation of single molecules. Acc. Chem. Res. 38, 523–533 (2005).
Miyauchi, K., Kozlov, M. M. & Melikyan, G. B. Early steps of HIV-1 fusion define the sensitivity to inhibitory peptides that block 6-helix bundle formation. PLoS Pathog. 5, e1000585 (2009).
Deeks, S. G., Smith, M., Holodniy, M. & Kahn, J. O. HIV-1 protease inhibitors. A review for clinicians. JAMA 277, 145–153 (1997).
Chojnacki, J. et al. Maturation-dependent HIV-1 surface protein redistribution revealed by fluorescence nanoscopy. Science 338, 524–528 (2012).
Binley, J. M. et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78, 13232–13252 (2004).
Clotet-Codina, I. et al. HIV endocytosis after dendritic cell to T cell viral transfer leads to productive virus infection. Antiviral Res. 83, 94–98 (2009).
Finnegan, C. M. et al. Sphingomyelinase restricts the lateral diffusion of CD4 and inhibits human immunodeficiency virus fusion. J. Virol. 81, 5294–5304 (2007).
Altenburg, J. D., Jin, Q., Alkhatib, B. & Alkhatib, G. The potent anti-HIV activity of CXCL12gamma correlates with efficient CXCR4 binding and internalization. J. Virol. 84, 2563–2572 (2010).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–795 (2006).
Bermudez-Hernandez, K. et al. A method for quantifying molecular interactions using stochastic modelling and super-resolution microscopy. Sci. Rep. 7, 14882 (2017).
Munro, J. B. et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 346, 759–763 (2014).
Kwon, Y. D. et al. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat. Struct. Mol. Biol. 22, 522–531 (2015).
Ma, X. et al. HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. eLife 7, e34271 (2018).
Tan, Q. et al. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 341, 1387–1390 (2013).
Qin, L. et al. Structural biology. Crystal structure of the chemokine receptor CXCR4 in complex with a viral chemokine. Science 347, 1117–1122 (2015).
Liu, Q. et al. Quaternary contact in the initial interaction of CD4 with the HIV-1 envelope trimer. Nat. Struct. Mol. Biol. 24, 370–378 (2017).
Wu, L. et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384, 179–183 (1996).
Yang, X. Z., Kurteva, S., Ren, X. P., Lee, S. & Sodroski, J. Subunit stoichiometry of human immunodeficiency virus type 1 envelope glycoprotein trimers during virus entry into host cells. J. Virol. 80, 4388–4395 (2006).
Nolan, R., Iliopoulou, M., Alvarez, L. & Padilla-Parra, S. Detecting protein aggregation and interaction in live cells: a guide to number and brightness. Methods 140–141, 172–177 (2018).
Padilla-Parra, S., Audugé, N., Coppey-Moisan, M. & Tramier, M. Quantitative FRET analysis by fast acquisition time domain FLIM at high spatial resolution in living cells. Biophys. J. 95, 2976–2988 (2008).
Sage, D., Neumann, F. R., Hediger, F., Gasser, S. M. & Unser, M. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans. Image Process. 14, 1372–1383 (2005).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Acknowledgements
This work has been supported by the Wellcome Trust to R.N. (105278) and G.M.J. (203852), Medical Research Council (MR/L009528/1) to T.A.B., and National Institutes of Health Oxford-Cambridge Fellowship to C.A.C. S.P.-P acknowledges funding from the Nuffield Department of Medicine leadership fellowship. All authors acknowledge funding from the Wellcome Trust Core Award (203141).
Author information
Authors and Affiliations
Contributions
S.P.-P conceived and supervised the study. M.I. and L.A. performed the experiments. R.N. produced the computational algorithms for image processing. M.I., R.N., L.A., C.A.C., G.M.J., and S.P.-P analyzed the data. Y.W. and T.A.B. built the atomic models. S.P.-P. wrote the manuscript with comments from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Cross number and brightness applied to detect CD4 and co-receptor interactions.
Cartoon depicting the fluorescence intensity fluctuation traces coming from two different channels (CD4 and co-receptors), that is, homotypic interactions. The amplitude of the fluorescence fluctuation is related to the oligomeric state of the proteins under study. The cross-variance analysis renders information about heterotypic interactions. If heterotypic interactions are detected, colocalization analysis between these regions and HIV-1 virions is performed. Subsequently, the oligomeric state for these regions is extracted, obtaining the time-resolved oligomeric state of the interaction upon addition of the HIV-1 virions on the surface of live cells.
Supplementary Figure 2 HIV-1 addition on COS7 cells expressing CD4-mOrange—CXCR4-mTFP1 does not induce positive FRET.
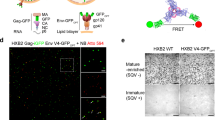
Fluorescence lifetime imaging microscopy (FLIM) was measured on COS7 cells expressing CD4-mOrange and CXCR4-mTFP1 before and after addition of HIV-1HXB2-Gag-iCherry virions. The CXCR4-mTFP1 lifetime remained the same (~2.4 ns), indicating that no measurable FRET occurred (bottom).
Supplementary Figure 3 HIV-1 virions induce a global positive Bcc on COS7 cells expressing CD4-mOrange—CXCR4-mTFP1.
a, An overall increase in brightness was seen in both channels (CD4 and CXCR4) all across the cell (second row) and in Bcc (last row) upon HXB2-HIV virion addition. Scale bar, 2 μm. b, Box plot diagram for the average oligomeric states for both CD4 (red dots) and CXCR4 (green dots) throughout the cell before and after addition of untreated HIV-1 (HXB2) virions or HIV-1 HXB2 virions treated with SQV (50 nM). c, Graph representing CD4 versus CXCR4 oligomeric states before (red dots) and after (green dots) HIV-1.
Supplementary Figure 4 CD4-mOrange and CXCR4-mTFP1 are mostly monomers and dimers before exposure to HIV-1 virions.
Box plot showing the average oligomeric state throughout the entire COS-7 cell surface for CD4-mOrange before and after HIV-1HXB2 (mostly monomers) virion addition (red dots) (mostly trimers) and CXCR4-mTFP1 (red dots) before (average monomers) and after HIV-1HXB2 (average dimers). The 0.05 level for one-way ANOVA shows that the population means are significantly different. b, Scatterplot showing the CD4–CXCR4 oligomeric states before (red dots) and after HIV-1HXB2 exposure. Each dot is the average oligomeric state taking the mean throughout the cells.
Supplementary Figure 5 b12 blocks HIV-1 infection and disrupts CD4–co-receptor interactions.
a, b12 titration curve showing the proportion of COS-7 cells expressing Gag-iCherry 24 h after HIVHXB2-Gag-iCherry exposure incubated with different concentrations of the antibody; the squares represent the average values, and the error bars denote the s.d. for n = 10 cells. b, Time-resolved oligomeric states for CXCR4-mTFP1 (green dots) and CD4-mOrange (red dots) following exposure to HIVHXB2-Gag-iCherry virions in the presence of 100 μg/mL b12. These virions did not induce positive Bcc. The circles represent the average values, and the whiskers denote the standard error for n = 12 events.
Supplementary Figure 6 HIV-1 virions treated with the protease inhibitor saquanivir do not induce CD4 and CXCR4 oligomerization nor positive Bcc.
a–c, COS-7 cells co-expressing CD4-mOrange and CXCR4-mTFP1 were imaged before and after exposure to HIVHXB2 virions (left panels). As shown before (Supplementary Fig. 3), these COS-7 cells show an overall increase in the CD4 oligomeric state (left panel in a) and a significant increase in positive Bcc (left panel in c). The 0.05 level for one-way ANOVA shows that the population means are significantly different. When treating the cells with 320 nM SQV (right panels), no significant increase in CD4 oligomerization or positive Bcc was observed (left panels in a and c). The 0.05 level for one-way ANOVA shows that the population means are not significantly different. In all cases, n = 10.
Supplementary Figure 7 Addition of CXCL12 and OKT4 inhibits both positive Bcc and HIV-1 fusion.
a–e, The addition of 100 nM CXCL12 (CXCR4 ligand) and OKT4 (50 μg/mL) blocks both time-resolved cross-variance signal (a–d) and the colocalization between HIVHXB2 and Bcc micrographs (e). f, Infection assays of COS-7 cells co-expressing CD4 and CXCR4 also show that productive infection is also blocked by 100 nM CXCL12 (CXCR4 ligand) and OKT4 (50 μg/mL). Scale bars, 300 nm.
Supplementary Figure 8 HIV-1 infection assays validate the use of COS-7 cells expressing labelled CD4 and co-receptors.
COS-7 cells (single images, left column; tiled images, right column) were exposed to HIVHXB2 virions (first row), HIVHXB2 virions treated with 350 nM saquanivir (SQV) (second row), HIVVSV-G virions (third row), no-Env HIV-1 virions (fourth row) and HIVJR-FL virions (fifth row). Expression of Gag-iCherry after 24 h only for infection with HXB2-, VSV-G- and JR-FL-decorated HIV-1 virions validates the use of these reporter cells. Scale bars, 1 μm (left column) and 10 μm (right column).
Supplementary Figure 9 Two-color dSTORM microscopy demonstrates a three-step mechanism for HIVHXB2 prefusion reaction.
a, Labeled CD4 and CCR5 receptors were labeled again with nanoboosters (Chromotek) specifically engineered for dSTORM imaging. b, HIVHXB2 virions were exposed to COS-7 cells and were fixed 10 min later and imaged in a TIRF-dSTORM setup equipped with two EM-CCD cameras (dual-cam; Methods). The prefusion reaction of individual HIV-1 virions was assessed by colocalization analysis of single molecules with a resolution of 30 nm per event. c, Colocalization-positive images were produced to generate masks to recover the average stoichiometry between CD4 and CXCR4 (average normalized sum of photons per interaction event) and the real stoichiometry (normalized sum of photons per interaction event). d, Histograms of average events for cells untreated with HIV-1 virions (left chart, black dots). The right plot corresponds to CD4-CXCR4 average events (proportion of interacting CD4 and CXCR4) from COS-7 cells exposed to HIV-1 virions decorated with HXB2 spikes. Scale bar, 300 nm.
Supplementary Figure 10 Number of Env molecules engaged to CD4 and co-receptors.
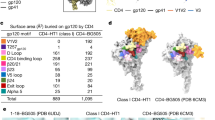
Histograms show the number of events of HIV-1 Env (JR-FL) (top) and HXB2 (bottom). Around one Env molecule seems to be engaged in CD4-CCR5 interactions, while two events (two distributions) are seen around 1 and 2 for HXB2 Env.
Supplementary Figure 11 b12 disrupts the interactions between CD4 and co-receptors.
Use of the interaction factor (IF) as defined in the Methods reveals that both CD4 and CXCR4 or CCR5 give similar IF values as untreated cells (first column). A t test to evaluate the average of interacting (n = 8) and non-interacting (n = 5) cells was performed between the first two columns from the left. At 0.05 confidence, the means are statistically different.
Supplementary Figure 12 In some instances, JR-FL Env engages with four CD4 molecules in step 3 before initiating the fusion reaction.
Four CD4 molecules can be engaged in the prefusion complex with 0.1 probability for JR-FL Env.
Supplementary information
Rights and permissions
About this article
Cite this article
Iliopoulou, M., Nolan, R., Alvarez, L. et al. A dynamic three-step mechanism drives the HIV-1 pre-fusion reaction. Nat Struct Mol Biol 25, 814–822 (2018). https://doi.org/10.1038/s41594-018-0113-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-018-0113-x
This article is cited by
-
Multicolor lifetime imaging and its application to HIV-1 uptake
Nature Communications (2023)
-
HIV-1 Env trimers asymmetrically engage CD4 receptors in membranes
Nature (2023)
-
Next-generation single virus tracking
Nature Methods (2022)