Abstract
Aging induces molecular, cellular and functional changes in the adult brain that drive cognitive decline and increase vulnerability to dementia-related neurodegenerative diseases. Leveraging systemic and lifestyle interventions, such as heterochronic parabiosis, administration of ‘young blood’, exercise and caloric restriction, has challenged prevalent views of brain aging as a rigid process and has demonstrated that aging-associated cognitive and cellular impairments can be restored to more youthful levels. Technological advances in proteomic and transcriptomic analyses have further facilitated investigations into the functional impact of intertissue communication on brain aging and have led to the identification of a growing number of pro-aging and pro-youthful factors in blood. In this review, we discuss blood-to-brain communication from a systems physiology perspective with an emphasis on blood-derived signals as potent drivers of both age-related brain dysfunction and brain rejuvenation.
Similar content being viewed by others
Main
The intimate relationship between the brain and cognition grounds the very perception of ourselves, and therefore it is not surprising that we often think of the brain as a separate entity all on its own, isolated from the interactions of the periphery. However, no other process may oppose this impression more strongly than aging itself, with its ever-present reminder as our hair grays, muscles weaken and memories become more distant. Indeed, aging is a process that affects all organs in the body, leading to substantial alterations in intertissue communication and regulation. Researchers have recently leveraged evolving proteomic approaches and single-cell RNA-sequencing technologies to begin to decode the functional impact of intertissue communication on brain aging1,2. The application of molecular approaches to investigate systemic and lifestyle interventions, such as heterochronic parabiosis (in which the circulatory systems of young and aged animals are surgically connected), young blood plasma administration, exercise and caloric restriction, has uncovered broad rejuvenating effects on the aged brain that are mediated through blood, which question the very notion that brain aging is immutable3,4,5,6,7,8,9,10. This review will first survey well-documented and emerging cellular hallmarks of brain aging, next categorize how bloodborne cellular and soluble signals drive brain aging and finally give an overview of the tissues, cells and downstream blood factors involved in transferring the benefits of systemic and lifestyle interventions to the brain. In this review, we refer to the process of restoring cognitive function and reversing cellular hallmarks of aging to a more youthful state as ‘rejuvenation’.
Impact of aging on the brain
The functional consequence of aging in the brain is the decline of cognitive faculties, such as memory loss, which at its core is driven by cellular and molecular changes. Here, we touch upon well-documented and emerging cellular hallmarks of brain aging with a focus on those amenable to pro-aging and rejuvenating interventions (Fig. 1)11,12,13. We differentiate between physiological aging and progression to neurodegenerative diseases, with the latter being characterized by selective neuronal cell loss, protein-aggregation pathology and additional molecular and cellular changes that each warrant their own review. Nevertheless, given that aging remains a prominent risk factor for age-related diseases such as Alzheimer’s disease (AD), systemic interventions that ameliorate cognitive and cellular function in aging may extend to neurodegenerative conditions.
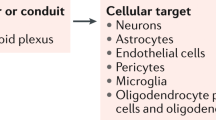
The figure shows cellular hallmarks of brain aging that have been investigated in the context of blood-based pro-aging and rejuvenating interventions. Hallmarks have been divided into four categories: functional changes of neurons and circuits (‘neuronal’), regenerative changes relating to adult NSCs and neurogenesis as well as OPCs and myelin renewal (‘regenerative’), inflammatory changes associated with microglia and astrocytes (‘inflammation’) and vasculature changes relating to the BBB (‘vasculature’). Abbreviations: ↓, decreased; ↑, increased; EC, endothelial cell; IEG, immediate early gene; NPC, neural progenitor cell; pCREB, phosphorylated CREB; RMT, receptor-mediated transport; ROS, reactive oxygen species. Red lightning bolts indicate inflammatory changes in BECs.
Neuronal dysfunction
A central hallmark of brain aging is maladaptive changes in the functional properties of neurons, reflected in decreased synaptic plasticity-related gene expression, reduced synaptic density and aberrant electrophysiological processes12,14,15. While overt signs of neuronal aging are not exhibited equally by all brain regions, some areas, including the hippocampus, are particularly susceptible to the effects of aging12. Functionally, age-related molecular and cellular changes can lead to both decreased and increased neuronal excitability, depending on the neuronal cell type and brain region16. The specificity of these changes suggests a complex dysregulation of excitatory and inhibitory inputs and broader maladaptive circuit changes that drive cognitive decline in processes such as spatial learning and memory, associative memory and episodic and working memory12,15,16.
Regenerative decline
Brain aging in mammals is characterized by a precipitous decline in regenerative capacity, mediated through changes in adult neuronal stem cells (NSCs) and oligodendrocyte progenitor cells (OPCs). Adult neurogenesis, the process through which NSCs give rise to new neurons, decreases dramatically across all three neurogenic regions17,18,19: the hippocampal dentate gyrus (DG), the subventricular zone (SVZ) lining the lateral ventricle and the hypothalamus20. Of note, the persistence of adult NSCs in the human DG remains controversial21,22,23. Neurogenesis regulates learning and memory in young adult rodents24,25; however, the functional impact of decreased neurogenesis on cognitive decline during old age remains unclear. By contrast, the number of OPCs distributed in gray and white matter remains relatively stable, although the rate of oligodendrocyte differentiation and myelin renewal is greatly decreased with age26. Age-related regenerative decline is mediated by both cell-intrinsic mechanisms and extrinsic changes to the neurogenic niche and systemic environment12,20.
Neuroinflammatory changes
An increasingly appreciated hallmark of brain aging is neuroinflammation. This process is predominantly mediated by microglia, the brain-resident macrophages. Microglial aging is characterized by increased production of reactive oxygen species, pro-inflammatory cytokines and components of the complement system27,28, extensive morphological changes and impairments in phagocytosis27,29,30. Functionally, age-related microglial phagocytic dysfunction has been linked to cognitive decline31. Microglia have also been suggested to play a critical role in astrocyte reactivity through secretion of pro-inflammatory cytokines and complement components in the aging brain32. In contrast to microglia, relatively few studies have focused on age-related functional changes of astrocytes32,33. With age, astrocytes become more ‘reactive’, characterized by upregulation of gene sets associated with synapse elimination, neurotoxicity and oligodendrocyte toxicity32,33,34. Both microglia and astrocytes are also thought to co-regulate, in part, synaptic pruning during aging through changes in molecular signals, such as complement29,30. There is growing appreciation for the functional heterogeneity of microglia and astrocytes across brain regions and developmental and adult time points. More broadly, neuroinflammatory changes may also be regulated by additional cell types such as border-associated macrophages and the diverse repertoire of immune cells residing in niches in the brain borders, raising the question of their roles as drivers of brain aging and cognitive decline35,36.
Vascular and blood–brain barrier changes
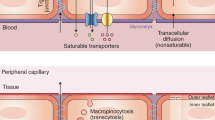
The vasculature in the brain forms a specialized blood–brain barrier (BBB), which regulates transport of nutrients, molecules and cells from the blood to the brain. Aging of brain vasculature is characterized by changes in vascular morphology and stiffness, dysregulation in cerebral blood flow (CBF) and tissue oxygenation37,38. Traditionally, it was considered that the BBB begins to break down with age, allowing leakage of molecules that can drive cognitive dysfunction39,40,41. Recently, advances in single-cell RNA-sequencing technologies have begun to elucidate more nuanced age-related changes in brain endothelial cells (BECs) and other cellular components of the brain vasculature42,43,44. BECs display region- and segment-specific increases in inflammatory and stress-response markers with age42,43,45, a reduction in density and volume and decreased pericyte coverage10,45. Experiments with labeled whole-blood plasma proteome in young animals revealed an astonishing amount of protein uptake by BECs, as well as by neurons and glia residing in the brain parenchyma44. This uptake of bulk plasma proteins was surprisingly reduced in aged mice, driven by pronounced changes in BEC transcytosis44. Although the exact composition of these transcytosed plasma factors remains unknown, the age-related changes coincide with a shift from ligand-specific receptor-mediated transport toward nonspecific caveolar transcytosis44. Age-related vascular and BBB changes fundamentally alter how signals from blood may be relayed to the brain; moreover, new findings focused on the choroid plexus (CP) blood–cerebrospinal fluid (CSF) barrier (Box 1) open new avenues to explore in our understanding of drivers of brain aging.
Bloodborne signals and the aging brain
Over the last decade, experiments using heterochronic parabiosis or heterochronic blood exchange from old to young animals have shown that these approaches induce a broad range of accelerated aging phenotypes in young animals8,10,46 (Fig. 2), positing components in aged blood as major drivers of dysfunction in the aging brain. Young mice exposed to blood factors and cells from the aged systemic milieu have decreased adult hippocampal and SVZ neurogenesis and reduced long-term potentiation (LTP), an indicator of synaptic transmission and a neuronal correlate of learning and memory8,10. Interestingly, administration of aged blood plasma alone similarly decreases neurogenesis, increases inflammatory states of microglia and BECs and impairs hippocampus-dependent cognitive functions in young recipient mice8,42,43. Recent work has begun to decode individual components in aged blood, including cells and soluble factors in plasma, which can recapitulate some of the detrimental effects of an aging systemic environment on the brain8,42,47,48,49.
Young mice are illustrated with brown coats, and aged mice are shown with gray coats. In heterochronic parabiosis, two mice are surgically connected for 4–6 weeks, so that a young animal is exposed to an aged systemic environment. In heterochronic blood exchange, approximately 50% of the blood (both cells and plasma) of a young mouse is replaced with an equal amount of blood derived from an aged mouse. The mice are not surgically connected. In aged plasma administration, plasma is collected from aged donor mice and intravenously delivered over the course of 3–4 weeks into young recipient mice. In aged HSC transplantation, the hematopoietic system of young recipient mice is reconstituted with HSCs derived from aged donor mice. Pro-aging effects have been assessed on neuronal, regenerative, neuroinflammatory and/or vascular functions in young mice. Abbreviations: ↔, no change; hipp, hippocampus. A question mark indicates limited supporting data.
Circulating immune cells and brain aging
The aging process leads to drastic changes in immune cell compositions and functions, resulting in increased local and systemic inflammation35. Under adult homeostatic conditions, the brain parenchyma is considered mostly devoid of peripheral immune cells. By contrast, the central nervous system (CNS) barrier regions, including the CP, meninges and skull bone marrow, are populated with a diverse repertoire of innate and adaptive immune cells36.
Aging myeloid cells display substantial cellular and functional changes associated with a maladaptive pro-inflammatory state and may be a potent source of pro-aging factors in the periphery and the CNS. In particular, prostaglandin E2 signaling through the EP2 receptor was demonstrated to be elevated in myeloid cells, impairing metabolic homeostasis and exacerbating pro-inflammatory signaling50. Conversely, inhibition of EP2 signaling selectively in peripheral myeloid cells was sufficient to reverse pro-inflammatory cytokine levels in plasma, restore hippocampal LTP and rescue cognition in aged mice50.
Parabiosis and bone marrow reconstitution trafficking studies indicate that only a limited number of infiltrating circulating immune cells are present in the aging brain parenchyma8,49. Similarly, post-mortem human histological analysis did not identify significant differences in the distribution and density of B cells and T cells in the cortex with age51. However, the possibility remains that small shifts in hematogenous immune cell populations within the brain may influence function during aging. Surprisingly, an increase in natural killer (NK) cells was recently reported in the hippocampus of aged humans and mice52. In aged rodents, it is suggested that NK cells expand locally in the DG and display elevated markers of cytotoxicity52. Conversely, ablation of NK cells enhanced neurogenesis and cognitive functions in aged mice52.
T cells have emerged as potential contributors to age-related brain dysfunction (Table 1). CD8+ T cells are increased in the SVZ of the lateral ventricle in aged mice and humans and drive impairments in adult neurogenesis through increased interferon-γ signaling53. Furthermore, T cells have been reported near sinuses and in the hippocampus of aged rodents45,54, and an increase in perivascular and parenchymal T cells was observed in white matter of aged rhesus macaques55. Although aging-associated T cell changes are relatively modest in the brain parenchyma, marked increases in T cells have been reported in rodent models of neurodegeneration and in CSF of human individuals with AD54,56. The extent to which immune cell populations act directly in the brain versus indirectly through interactions with the brain vasculature or borders or secreted systemic factors requires further investigation in both aging and neurodegeneration.
Pro-aging blood factors as drivers of brain dysfunction
Experiments involving heterochronic parabiosis and aged plasma administration (Fig. 2) indicate that the detrimental effects of aged blood can, in part, be mimicked by circulating pro-aging blood factors. Using proteomic approaches, a series of blood factors in plasma have been identified, which increase with age and promote features of aging in the brain. Here, we highlight pro-aging blood factors that recapitulate age-related cellular and cognitive dysfunction when administered to young individuals (Table 1).
Cytokines and chemokines
Cytokines and chemokines (secreted proteins that broadly regulate immune responses and cell trafficking) have emerged as prominent pro-aging factors with neuro-modulatory properties57. Several members of the C–C chemokine family were shown to be increased in blood plasma of aged rodents and in young heterochronic parabionts exposed to an aged systemic environment. CCL11 or eotaxin-1 increases in plasma and CSF of rodents and humans8,58. Systemic treatment with CCL11 was shown to reduce hippocampal neurogenesis, activate microglia and impair cognitive function in mice8,59,60. Radiolabeling experiments indicate that CCL11 can cross the BBB in rodents61. Moreover, the negative effects of systemic CCL11 on neurogenesis can be blocked with central delivery of a neutralizing antibody, suggesting that CCL11 acts locally in the hippocampus8. Interestingly, the CCL11 gene is located in a genomic region with additional C–C motif genes including CCL2 (MCP-1), the product of which is similarly elevated in blood plasma of aged mice and young heterochronic parabionts8. CCL2 has been suggested to contribute to BBB dysfunction and permeability62. In humans, the levels of CCL11 and CCL2 are associated with cognitive decline in individuals with mild cognitive impairments or AD and negatively correlate with memory functions63,64, suggesting that their detrimental effects on cognition may be conserved across species.
Components of MHC-I
Systemic levels of β2-microglobulin (B2M), the soluble light chain of major histocompatibility complex class I (MHC-I) molecules, are elevated in aged mice and in the plasma and CSF of older humans8,48. Mimicking this aging-associated increase through systemic administration of B2M decreases adult hippocampal neurogenesis and impairs cognitive functions in mice. In humans, B2M levels are increased in patients with AD and human immunodeficiency virus (HIV)-associated dementia65,66, indicating a conserved association with impaired cognition. Interestingly, B2M and MHC-I genes are upregulated in various cell types in the aged brain32,43,67, and direct delivery of B2M into the hippocampus also impairs neurogenesis and cognition. These detrimental effects were abrogated in mice that lack surface expression of classical MHC-I, indicating that its molecular effect may depend on the specific repertoire of MHC-I molecules expressed on individual cell types in the brain67,68,69. Aged mice lacking B2M display increased neurogenesis and enhanced cognition, suggesting that targeting B2M may be a viable therapeutic avenue to restore function to the aged brain48. Notably, transforming growth factor (TGF)-β1 was identified as a potential regulator of B2M expression in both the periphery and the CNS47. Systemic inhibition of TGF-β1 signaling reduced B2M levels and enhanced adult neurogenesis in aged mice. Thus TGF-β1 and B2M may act through converging pathways to promote regenerative decline in the aging brain47.
Hematopoietic system-induced factors
Aging-associated changes of the immune system together with the role of pro-aging immune factors in promoting brain dysfunction point to the aging hematopoietic system as a driver of brain aging. Inflammatory insults have been shown to induce a persistent increase in markers of hematopoietic stem cell (HSC) aging even in young mice70,71. Moreover, heterochronic HSC transplantations (in which the bone marrow of young mice is reconstituted with old HSCs) reduced synaptic density, decreased hippocampal neurogenesis and impaired cognitive functions in adult recipient mice49 (Fig. 2). Mass spectrometry analysis identified several putative pro-aging plasma factors associated with an aging hematopoietic system in the heterochronic HSC transplantation model. Secreted cyclophilin A (CyPA) was negatively correlated with cognitive performance in mice. Elevating systemic levels of CyPA impaired cognition in young mice, whereas neutralizing antibody treatment enhanced neuronal and cognitive functions in aged mice49. These findings suggest that neutralization of individual components of aging blood may provide a viable therapeutic strategy to rejuvenate the aged brain. Although the cellular source of systemic CyPA in aging remains unknown, systemic administration of CyPA has been shown to act on BECs, suggesting that it may indirectly modulate brain functions by targeting the brain vasculature and BBB integrity72.
Vasculature-derived factors
Several recent discoveries establish the brain vasculature as a potential source of pro-aging factors. Vascular cell adhesion molecule 1 (VCAM1) is a membrane protein that is predominantly expressed on BECs in venous and arterial segments of the brain vasculature42. VCAM1 expression is upregulated in response to inflammatory stimuli such as an aging systemic milieu and facilitates interactions of leukocytes with the vasculature42,43. As a result of shedding, soluble VCAM1 levels are elevated in the plasma of aged mice and humans42. Additionally, circulating VCAM1 is elevated in patients with AD, and elevated VCAM1 levels are associated with cognitive impairments73,74. Treatment of aged mice with a VCAM1-neutralizing antibody or genetic ablation of Vcam1 selectively in BECs increased hippocampal neurogenesis, reduced microglial activation and enhanced cognitive function42. Thus, endothelial VCAM1 is, at least in part, involved in relaying certain aging-associated signals from the periphery to the brain parenchyma.
Acid sphingomyelinase (ASM) is a sphingolipid-metabolizing enzyme secreted by endothelial cells75. Similar to VCAM1, ASM expression is elevated in the aging brain vasculature and the levels of secreted ASM are increased in mouse and human plasma with age. Normalizing ASM levels through genetic or targeted knockdown approaches restored vessel density and caveolar transcytosis toward youthful levels, increased synaptic spine number in the hippocampus and improved cognitive function in aged mice. Together, VCAM1 and ASM point to BECs as a critical source of systemic pro-aging factors and further establish the brain vasculature as a critical nexus in blood-to-brain communication during aging.
Risk factors, co-morbidities and the aging brain
Risk factors and co-morbidities linked to neurodegenerative diseases, including obesity, diabetes, hypertension, cardiovascular disease and chronic inflammation, are associated with decreased cognitive performance in older people and in animal models38,76,77. For example, animal models of obesity display impaired synaptic plasticity, increased neuroinflammation and reduced hippocampal neurogenesis77,78. Similarly, chronic and acute inflammatory conditions, such as long coronavirus disease 2019 (COVID-19) with brain fog and animal models of respiratory COVID-19 infections, were shown to recapitulate neuroinflammatory, regenerative and cognitive changes that mirror the previously described hallmarks of brain aging60. Moreover, the levels of plasma CCL11, originally described as a pro-aging factor in heterochronic parabiosis, are elevated in animal models and humans with COVID-19 (ref. 60). The link between risk factors, co-morbidities and memory impairments suggests a complex pathophysiology that may potentiate cellular and functional changes associated with brain aging and warrants further investigations. Thus, therapeutic or lifestyle interventions targeting risk factors and co-morbidities may directly or indirectly restore function to the aged brain by engaging pro-aging systemic drivers.
Systemic rejuvenating interventions
A growing body of work has investigated the effect of ‘young blood’ on the aging brain and whether a more youthful systemic milieu can ameliorate age-related brain dysfunction8,9,10. Similarly, lifestyle interventions, including exercise and caloric restriction, rejuvenate the aged brain3,5,7,79. These systemic and lifestyle interventions demonstrate that there is latent plasticity in the aged brain that can be harnessed to improve cognitive function late in life. Here, we summarize the various rejuvenating effects ascribed to each systemic intervention (Fig. 3).
Interventions are categorized into blood-based and lifestyle interventions. Young mice are illustrated with brown coats, and aged mice are shown with gray coats. Blood-based interventions: in heterochronic parabiosis, an aged mouse is surgically connected to a young mouse for 4–6 weeks and is exposed to a youthful systemic environment. In young plasma administration, the plasma fraction is collected from young donor mice and intravenously delivered to aged recipient mice over the course of 3–4 weeks. In neutral blood exchange, approximately 50% of the plasma is removed from aged mice and replaced with saline and albumin. In young bone marrow transplantation, the immune system of aged recipient mice is reconstituted with bone marrow cells derived from young donor mice. Lifestyle interventions: physical exercise paradigms can be of different duration and intensity. Caloric restriction paradigms are dietary interventions in which caloric intake is decreased by 10–50% without malnutrition. Rejuvenating effects have been assessed on neuronal, regenerative, neuroinflammatory and/or vascular functions in aged mice.
Blood-based interventions
Heterochronic parabiosis has been shown to rejuvenate aged muscle, liver, heart, pancreas, bone, spinal cord and brain, ultimately leading to an extension of life and healthspan9,10,80,81,82,83,84. In the brain, it ameliorates multiple cellular hallmarks of aging, resulting in increased synaptic plasticity and synaptic density9, increased hippocampal and SVZ neurogenesis9,10, increased vascular density and CBF10 and attenuation of cellular senescence markers within the forebrain85. Likewise, restoring the cellular component of aged blood with young immune cells through heterochronic bone marrow transplantation increased synaptic density, attenuated microglial activation and enhanced hippocampus-dependent cognitive function in aged mice59 (Fig. 3).
Multiple reports have shown systemic administration of the plasma component of young blood to be effective at reversing hippocampus-dependent cognitive and regenerative dysfunction in aged mice9,71,86 (Fig. 3), at least in part by enhancing synaptic plasticity through activation of the transcription factor cAMP response element-binding protein (CREB)9. Similarly, diluting plasma of aged mice by approximately 50% with a saline–albumin solution, through a procedure known as neutral blood exchange, enhanced regenerative capacity, mitigated microglial activation and improved cognition87,88 (Fig. 3). Moreover, dilution of aged blood by neutral blood exchange enabled elevation of plasma factors known to promote brain health and function, suggesting that the aging systemic milieu may dampen the abundance of potential pro-youthful factors88. Young blood and plasma exchange-based approaches are now being tested in pre-clinical and clinical studies for aging-associated neurodegenerative diseases such as AD89,90. Collectively, these blood-based rejuvenating interventions highlight the importance of both administering pro-youthful factors and blocking or diminishing pro-aging factors as complementary potential therapeutic approaches to reverse age-related dysfunction in the brain.
Physical activity and exercise
Physical activity and aerobic exercise mitigate age-related cognitive dysfunction in rodents3,7,91, non-human primates92 and humans4,93. Broad cellular and molecular hallmarks of brain aging are improved by exercise (Fig. 3). Several groups have reported benefits on vascular aging, including increased vascular density94 and pericyte coverage of BECs, as well as reduced vascular leakage41. The benefits of exercise extend to cell types residing in the parenchyma. Exercise has been shown to increase synaptic plasticity41,95, enhance adult hippocampal neurogenesis3,7,91 and mitigate neuroinflammation by reducing microglial and astrocytic reactivity41,91,96. Furthermore, recent work has demonstrated that blood plasma from ‘exercised’ mice can transfer the beneficial effect of exercise on the brain and cognitive function to young and aged mice and mouse models of AD pathology7,96. Many of these positive exercise-induced effects observed in the brain have been suggested to be mediated, at least in part, by induction of the neurotrophin brain-derived neurotrophic factor (BDNF)97.
Caloric restriction
Caloric restriction, in which caloric intake is decreased by 10–50% without malnutrition, has been demonstrated to enhance learning and memory of aging rodents79,98, non-human primates99 and humans100. Caloric restriction paradigms have been shown to maintain white and gray matter integrity in the aging brain while preserving CBF79,101,102. In the parenchyma, caloric restriction of aged individuals promoted synapse formation and other markers of synaptic plasticity98,103, increased neurogenesis104 and attenuated markers of neuroinflammation103,105 (Fig. 3). Many of these beneficial effects of caloric restriction on the aged brain have been credited to advantageous metabolic alterations, protection from oxidative stress, neurotrophic factor production and increased autophagy. Additionally, caloric restriction may also have indirect benefits on the aged brain by reducing risk factors and co-morbidities associated with cognitive impairments98,102,106.
Bloodborne factors as drivers of brain rejuvenation
Following the discovery that administration of plasma derived from young or ‘exercised’ animals rejuvenates the aged brain7,9,86, multiple groups have attempted to identify a pro-youthful factor responsible for the effect of ‘young blood’ and lifestyle interventions on the aged brain by assessing changes in the composition of blood. The multifaceted effects of blood on the aged brain are highlighted by the growing number of beneficial factors identified and by the varied mechanisms by which they exert their rejuvenating effects, discussed below (Table 2).
Young bloodborne rejuvenating factors
One of the first reported rejuvenating factors in young blood was growth differentiation factor 11 (GDF11)83. As a member of the activin–TGF-β superfamily of growth and differentiation factors, GDF11 was originally identified as a mediator of skeletal patterning during embryogenesis107. Although the age-related dynamics of its abundance in blood are under debate83,108, GDF11 reproduced some of the beneficial effects of heterochronic parabiosis on the aged brain by increasing vascular density and CBF, ultimately leading to enhanced adult neurogenesis in the SVZ and the DG and increased markers of neuronal activity in the hippocampus and cortex10,109. Surprisingly, increased GDF11 expression within the adult hippocampus inhibits neurogenesis110. These disparate findings were reconciled when biotinylation experiments determined that circulating GDF11 is unable to cross the BBB109, suggesting that systemic GDF11 exerts its rejuvenating effect on the aged brain by signaling through BECs.
By contrast, a second young blood-derived rejuvenating factor, the bone-derived hormone osteocalcin, is capable of crossing the BBB111. When administered systemically, it can bind to neurons, increase BDNF levels, promote action potential in excitatory neurons and enhance learning and memory in aged mice86,111. Interestingly, antibody-based depletion of osteocalcin demonstrated its necessity for the beneficial effect of young blood plasma on hippocampal cognitive function in aged mice86.
To identify potential human rejuvenating factors, others discovered that systemic administration of human umbilical cord plasma rejuvenates the brain of aged immunodeficient mice112. Within human umbilical cord plasma, tissue inhibitor of metalloproteinases 2 (TIMP2) and colony-stimulating factor 2 (CSF2 or granulocyte–macrophage colony-stimulating factor (GM-CSF)) were identified as two factors that decrease by early adulthood112. Autoradiographic labeling of TIMP2 (ref. 112) and CSF2 (ref. 113) revealed both factors to be BBB permeable. When injected systemically, both factors were sufficient to enhance synaptic plasticity and hippocampus-dependent memory in aged mice112. CSF2 also restored cognition in a mouse model of AD pathology114.
Additional work attempted to screen for potential pro-youthful factors capable of acting on human neurons by treating human induced neurons in vitro with factors found to be downregulated with age in mouse serum115. This work revealed two factors capable of promoting synaptogenesis and dendrite arborization: the secreted matricellular proteins thrombospondin 4 (THBS4) and SPARC-like protein 1 (SPARCL1)115. Interestingly, lower plasma levels of THBS4 were found to be associated with impaired long-term recall in asymptomatic middle-aged humans from families with autosomal dominant AD116. However, it is unknown whether THBS4 or SPARCL1 are beneficial when administered systemically or whether they can cross the BBB to act directly on neurons.
Complementary approaches have also been used to identify circulating factors that can rejuvenate the aged brain. Circulating levels of the pro-longevity factor α-klotho do not change with age117; however, peripheral administration of a fragment of α-klotho enhances synaptic plasticity and cognition in aged mice118. Although α-klotho does not seem to cross the BBB, as shown in tagging experiments119, one report suggests that it may be regulating inflammation at the CP blood–CSF barrier120. Along similar lines, inflammation within the aged hypothalamus has been shown to inhibit expression of gonadotropin-releasing hormone (GnRH), a hypothalamic neuropeptide responsible for regulating reproductive biology, and drive aging phenotypes in mice19. Interestingly, peripheral administration of GnRH is sufficient to enhance hippocampal neurogenesis and restore cognitive function in aged rodents19. Systemic GnRH may be targeting the CNS through the circumventricular organs, specialized parts of the brain vasculature lacking a canonical BBB19.
Physical activity and exercise-induced factors
It has long been appreciated that, in response to acute and/or chronic aerobic exercise, humoral factors termed exerkines121 are released from skeletal muscle and other metabolically active tissues such as liver and adipose tissue and are able to mediate some of the beneficial effects of exercise (Table 2).
One of the first exerkines shown to enhance brain function in aged animals was insulin-like growth factor 1 (IGF1). Plasma IGF1 levels increase acutely during aerobic exercise122, and radiolabeling experiments revealed that IGF1 is able to cross the BBB via receptor-mediated transport123. Systemic IGF1 promotes markers of synaptic plasticity124 and neurogenesis125 and ameliorates age-related cognitive dysfunction126. Moreover, systemically blocking the receptor for IGF1, at least in young rats, was sufficient to abolish the effect of voluntary running on BDNF production in the hippocampus and spatial memory127, suggesting that some of the beneficial effects of exercise on the brain are promoted by systemic IGF1.
Recent work has begun to highlight a liver-to-brain axis by which liver-derived blood factors transfer the benefits of exercise to the aged brain. Both aged mice exposed to exercise for 6 weeks and physically active older humans have elevated blood levels of glycosylphosphatidylinositol (GPI)-specific phospholipase D1 (GPLD1 or GPI-PLD), a liver-derived protein that cleaves GPI-anchored proteins from the plasma membrane7. Additionally, liver and plasma GPLD1 levels were recently reported to be elevated in long-lived mutant mouse strains and mice treated with lifespan-extending drugs128. Increasing liver-derived systemic GPLD1 levels in aged mice recapitulated the benefit of exercise on hippocampal neurogenesis and cognitive function7. HiBiT-tagged bioluminescence experiments indicate that GPLD1 does not readily cross the BBB, and mass spectrometry analysis implicates changes in coagulation and complement signaling cascades downstream of GPI-anchored substrate cleavage as possible mediators of the rejuvenating effects7. Another predominantly liver-derived protein, selenoprotein P (SEPP1), is also upregulated in blood of mice after 4 d of voluntary wheel running129. SEPP1 transports selenium across the BBB by binding LDL-like receptor 8 (LRP8), and both SEPP1 and LRP8 are required for the exercise-induced increase in hippocampal neurogenesis129. Additionally, selenium supplementation was found to increase hippocampal neurogenesis and reverse cognitive dysfunction in aged mice129. This emerging body of work highlights the liver as a potential hub of exercise-induced rejuvenation, capable of influencing the systemic milieu to ameliorate age-related brain dysfunction.
Recently, clusterin (or apolipoprotein J (ApoJ)), also an LRP8 ligand and predominantly expressed in hepatocytes and cardiomyocytes96,130, was identified as an exerkine following 4 weeks of exercise with implications for neuroinflammation and AD. Clusterin is a multifunctional apolipoprotein that acts as an inhibitor of apoptosis, inflammation and complement activation96,131. Systemically administered clusterin binds to LRP8 on BECs and reduces inflammation following lipopolysaccharide treatment in young mice as well as in a mouse model of AD pathology96. Additional exercise-induced blood factors have also been demonstrated to improve brain function in the context of AD. Skeletal muscle-derived circulating levels of irisin, a BBB-permeable protein132 released upon cleavage from the transmembrane protein fibronectin type III domain-containing protein (FNDC5), are increased in response to 3 weeks of wheel running in mice133 and 12 weeks of high-intensity aerobic training in humans134. Interestingly, levels of FNDC5 and irisin are decreased in the brains of individuals with AD. In mouse models of AD pathology, both peripheral and central overexpression of Fndc5 or irisin has been shown to enhance synaptic plasticity, reduce glial activation and improve cognitive performance, whereas antibody-mediated blockade of FNDC5 diminishes the beneficial effect of voluntary wheel running on synaptic plasticity and memory132,135. Although levels of FNDC5 and irisin have been reported to be increased in the aged CSF, it is still unknown whether the benefits of irisin extend to aging-associated cognitive deficits135.
A number of additional exerkines, including vascular endothelial growth factor (VEGF)136, lactate137, cysteine protease cathepsin B (CTSB)138, platelets and platelet factor 4 (PF4)139,140, have been identified in young animals, but their effect on the aged or degenerating brain has yet to be investigated (Table 2).
Caloric restriction signals
In contrast to young blood and exercise interventions, potential systemic mediators of the rejuvenating capacity of caloric restriction on the aged brain have not been extensively examined. However, a number of studies have reported an increase in ketone body levels in calorically restricted animals79,141. Ketone bodies, consisting of acetone, acetoacetate and β-hydroxybutyrate, are metabolites produced during fatty acid metabolism in the liver, can be transported throughout the body and serve as a glucose-sparing energy source. Supplementation with ketone esters and dietary manipulations to augment endogenous ketone production attenuate memory loss in aging142,143,144 and in mouse models of neurodegenerative diseases145. Further exploration is needed to understand the capacity of ketone bodies to reverse hallmarks of brain aging. Likewise, work identifying additional circulating blood factors capable of conferring the benefits of caloric restriction to the aged brain could have a profound effect on developing therapies for age-related brain dysfunction.
Conclusions and future directions
Applying cutting-edge molecular technologies to investigate effects of systemic and lifestyle interventions has yielded insight into the cellular and molecular targets and tissues of origin of pro-aging and pro-youthful factors in blood. A number of these circulating factors have been posited for future therapeutic applications to enhance cognitive resilience and reduce risk for dementia-related neurodegenerative diseases. However, to achieve this promise, it is critical for the burgeoning field of brain rejuvenation to tackle a series of important questions that remain unanswered.
To what extent do pro-aging and pro-youthful factors act through convergent or divergent mechanisms? With respect to a common tissue of origin, the hematopoietic system and inflammatory processes emerge as a source of pro-aging factors. Nevertheless, in many cases, the cell type or tissue sources remain obfuscated. Although earlier work identified a series of muscle-derived myokines, the liver as a major secretory organ is rapidly emerging as an additional source of exercise-induced factors, with IGF1, GPLD1, SEPP1 and clusterin all being putative liver-derived exerkines (Fig. 4). Regarding mechanisms of action, numerous aging and rejuvenating factors exert similar effects on the brain; therefore, it is important to understand whether each factor acts through the same or parallel cellular targets and molecular pathways. Given the predominant immune nature of pro-aging factors in old blood, microglia appear an obvious first target. However, several recent studies are highlighting BECs as a potential nexus by which pro-aging factors, including VCAM1, ASM, CyPA and CCL2, regulate brain aging. Conversely, pro-youthful factors identified across interventions, such as GDF11, clusterin, GPLD1 and α-klotho, may likewise exert their rejuvenating effects indirectly on the aged brain by restoring function to the aging vasculature and additional peripheral targets. Additionally, a series of pro-youthful factors, including TIMP2, osteocalcin, SPARCL1 and THSB4, appear to selectively enhance synaptic or cognitive functions; whereas others, such as FGF17 and SEPP1, have been demonstrated to regulate regenerative and stem cell functions. Collectively, these findings indicate that brain function can be restored through several parallel targets as well as direct and indirect mechanisms with relevance for future therapeutic approaches.
Systemic factors and cell types, their potential tissue of origin and direct versus indirect mechanisms of action on functional hallmarks of brain aging are divided into three main categories: youthful and longevity factors (a), factors associated with systemic (or lifestyle) interventions such as exercise and caloric restriction (b) and pro-aging factors (c). a, Youthful and longevity factors (indicated in brown) are of undetermined origin. TIMP2, CSF2, α-klotho, THBS4, SPARCL1 and osteocalcin (OCN) enhance synaptic and/or regenerative functions directly in the aged brain. GDF11 and α-klotho act through potentially indirect mechanisms (for example, by enhancing brain vascular function). THBS4 and SPARCL1 enhance neuronal functions in vitro but have not been tested in vivo. The effect of pro-youthful factors on neuroinflammation has not been tested. b, Exercise-induced factors (exerkines, indicated in blue) are predominantly derived from muscle (myokines: FNDC5 and irisin) and liver (hepatokines: IGF1, GPLD1, SEPP1, clusterin (Clu)) and enhance synaptic and regenerative functions during old age. c, Pro-aging factors (indicated in red) are predominantly immune-related molecules, such as cytokines and chemokines (CCL11, CCL2, B2M) and immune cells (T cells and NK cells). Pro-aging factors drive maladaptive neuroinflammatory changes, inhibit neurogenesis and impair synaptic plasticity in the brain. A question mark indicates unknown effect or limited supporting data; a dashed line indicates a potentially indirect mechanism; an asterisk indicates an unknown tissue or cell source; an arrowhead indicates a promotion; and a flathead represents inhibition of a cellular process in the brain.
How broad ranging are the effects of pro-aging and pro-youthful factors throughout the brain? Apart from GDF11, most pro-aging and pro-youthful factors have been investigated in a single brain area or in the context of specific cellular hallmarks of brain aging, such as neurogenesis or synaptic plasticity. Considering the differential effects of aging on different brain regions and a growing understanding of the cellular and regional heterogeneity of neurons, microglia and astrocytes, it is essential that the effect of each circulating blood factor be examined across a wider range of brain regions and cellular hallmarks of brain aging.
Are there additional unidentified blood factors? Identification of pro-aging and pro-youthful blood factors has been challenging, as both mass spectrometry and antibody- or aptamer-based technologies have their own limitations in the total number of factors that can be surveyed, as well as biases in the factors that are enriched for by each platform. One such confound is that the protein content in blood plasma is often obscured by highly abundant proteins such as albumin and globulins, often requiring depletion methods for downstream analyses and detection of the larger number of the less-abundant blood protein components. Additional rejuvenating blood factors may be membrane bound or located within microvesicles, exosomes or platelets and released only in specific cellular contexts. This highlights the need for new tools to detect potential unidentified blood factors, for example, bioorthogonal labeling approaches. This will enable broader and more refined assessment of intertissue communication between peripheral signaling hubs, such as the liver, and the aging or rejuvenated brain. Additionally, unidentified blood factors could be of a completely different nature from those reported thus far, including lipids and metabolites. This consideration will be important as research begins to address whether the effects of other interventions, such as hormetic stresses and caloric restriction, can be transferred through systemic administration of circulating blood factors.
What would potential therapeutic strategies leveraging pro-youthful and pro-aging factors look like? It is unlikely that a single factor drives aging or that a single therapeutic intervention would be sufficient to restore function to a whole organism across all tissues. Because individuals appear to progress along different aging trajectories, future therapies would probably necessitate a combination of biomarker analyses to decode the unique aging profile in combination with a personalized treatment regimen composed of lifestyle interventions, pro-youthful factors and inhibition of pro-aging factors. For example, treatment may consist of mimetics of pro-youthful factors that decrease with age (for example, GDF11 or osteocalcin) and inhibition of pro-aging factors that increase with age (for example, CCL11 or B2M). These interventions may be synergistically combined with aging-independent pathways, such as exerkine mimetics (for example, GPLD1 or SEPP1) that enhance cognitive function but with levels that do not change with age7,118. Furthermore, identification of convergent mechanisms may point to future molecular targets, activation of which may provide additive benefits of multiple systemic factors.
Ultimately, rapid advances in our understanding of the tissues, cells and circulating blood factors involved in mitigating drivers of brain aging or transferring the benefits of systemic and lifestyle interventions bring hope that one day the memories typically lost with age can instead remain grounded in the rejuvenated plasticity that is unlocked through the intricate communication between blood and brain.
References
Lehallier, B. et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 25, 1843–1850 (2019). This is an extensive resource of aging-associated changes of the human plasma proteome.
Schaum, N. et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Praag, H., van, Shubert, T., Zhao, C. & Gage, F. H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 (2005).
Kramer, A. F. et al. Ageing, fitness and neurocognitive function. Nature 400, 418–419 (1999).
Ingram, D. K., Weindruch, R., Spangler, E. L., Freeman, J. R. & Walford, R. L. Dietary restriction benefits learning and motor performance of aged mice. J. Gerontol. 42, 78–81 (1987).
Witte, A. V., Fobker, M., Gellner, R., Knecht, S. & Flöel, A. Caloric restriction improves memory in elderly humans. Proc. Natl Acad. Sci. USA 106, 1255–1260 (2009).
Horowitz, A. M. et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020). The beneficial effects of exercise during old age can be transferred through blood plasma and the liver-derived systemic enzyme GPLD1.
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011). This report demonstrates that systemic blood factors, including CCL11, impair cognitive and regenerative functions.
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014). This study provided evidence that blood plasma administration is sufficient to restore cognitive deficits in aged recipient mice.
Katsimpardi, L. et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 (2014). GDF11 treatment enhances vascular function and neurogenesis in the aged SVZ.
Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 (2016).
Fan, X., Wheatley, E. G. & Villeda, S. A. Mechanisms of hippocampal aging and the potential for rejuvenation. Annu. Rev. Neurosci. 40, 251–272 (2017).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581 (2019).
Wheatley, E. G. et al. Neuronal O-GlcNAcylation improves cognitive function in the aged mouse brain. Curr. Biol. 29, 3359–3369 (2019).
Mattson, M. P. & Arumugam, T. V. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 27, 1176–1199 (2018).
Simkin, D. et al. Aging-related hyperexcitability in CA3 pyramidal neurons is mediated by enhanced A-type K+ channel function and expression. J. Neurosci. 35, 13206–13218 (2015).
Gontier, G. et al. Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep. 22, 1974–1981 (2018).
Enwere, E. et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365 (2004).
Zhang, G. et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 (2013).
Negredo, P. N., Yeo, R. W. & Brunet, A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 27, 202–223 (2020).
Sorrells, S. F. et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381 (2018).
Boldrini, M. et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589–599 (2018).
Moreno-Jiménez, E. P. et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560 (2019).
Akers, K. G. et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014).
Sahay, A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011).
Wang, F. et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 23, 481–486 (2020).
Sierra, A., Gottfried‐Blackmore, A. C., McEwen, B. S. & Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424 (2007).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Stephan, A. H. et al. A dramatic increase of C1q protein in the CNS during normal aging. J. Neurosci. 33, 13460–13474 (2013).
Shi, Q. et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J. Neurosci. 35, 13029–13042 (2015).
Pluvinage, J. V. et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568, 187–192 (2019).
Clarke, L. E. et al. Normal aging induces A1-like astrocyte reactivity. Proc. Natl Acad. Sci. USA 115, E1896–E1905 (2018).
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N. & Allen, N. J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285 (2018).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Buckley, M. W. & McGavern, D. B. Immune dynamics in the CNS and its barriers during homeostasis and disease. Immunol. Rev. 306, 58–75 (2022).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Silva, T. M. D. & Faraci, F. M. Contributions of aging to cerebral small vessel disease. Annu. Rev. Physiol. 82, 275–295 (2019).
Ungvari, Z. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 15, 555–565 (2018).
Montagne, A. et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Nation, D. A. et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019).
Soto, I. et al. APOE stabilization by exercise prevents aging neurovascular dysfunction and complement induction. PLoS Biol. 13, e1002279 (2015).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25, 988–1000 (2019). This study characterizes aging BECs and the effect of VCAM1 on neuroinflammatory, regenerative and cognitive dysfunction with age.
Chen, M. B. et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 30, 4418–4432 (2020).
Yang, A. C. et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430 (2020). This study used a new and innovative approach to investigate BBB function and plasma protein transport with age.
Propson, N. E., Roy, E. R., Litvinchuk, A., Köhl, J. & Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood–brain barrier permeability during aging. J. Clin. Invest. 131, e140966 (2021).
Rebo, J. et al. A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood. Nat. Commun. 7, 13363 (2016).
Yousef, H. et al. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget 6, 11959–11978 (2015).
Smith, L. K. et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 21, 932–937 (2015).
Smith, L. K. et al. The aged hematopoietic system promotes hippocampal‐dependent cognitive decline. Aging Cell 19, e13192 (2020).
Minhas, P. S. et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 590, 122–128 (2021). Targeting EP2 signaling in aged peripheral myeloid cells reduces systemic and brain inflammatory states and enhances synaptic plasticity and cognitive functions in aged animals.
Berry, K. et al. B and T lymphocyte densities remain stable with age in human cortex. ASN Neuro 13, 17590914211018116 (2021).
Jin, W.-N. et al. Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat. Neurosci. 24, 61–73 (2021).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019). This study identified a role for infiltrating immune cells on neuronal stem cell function in the aging SVZ.
Unger, M. S. et al. CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav. Immun. 89, 67–86 (2020).
Batterman, K. V., Cabrera, P. E., Moore, T. L. & Rosene, D. L. T cells actively infiltrate the white matter of the aging monkey brain in relation to increased microglial reactivity and cognitive decline. Front. Immunol. 12, 607691 (2021).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020).
Ransohoff, R. M. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31, 711–721 (2009).
Baruch, K. et al. CNS-specific immunity at the choroid plexus shifts toward destructive TH2 inflammation in brain aging. Proc. Natl Acad. Sci. USA 110, 2264–2269 (2013).
Das, M. M. et al. Young bone marrow transplantation preserves learning and memory in old mice. Commun. Biol. 2, 73 (2019). This study used heterochronic bone marrow transplantation to rejuvenate the aged brain.
Fernández-Castañeda, A. et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185, 2452–2468 (2022).
Erickson, M. A., Morofuji, Y., Owen, J. B. & Banks, W. A. Rapid transport of CCL11 across the blood–brain barrier: regional variation and importance of blood cells. J. Pharmacol. Exp. Ther. 349, 497–507 (2014).
Stamatovic, S. M. et al. Monocyte chemoattractant protein-1 regulation of blood–brain barrier permeability. J. Cereb. Blood Flow Metab. 25, 593–606 (2004).
Bettcher, B. M. et al. MCP-1 and eotaxin-1 selectively and negatively associate with memory in MCI and Alzheimer’s disease dementia phenotypes. Alzheimers Dement. 3, 91–97 (2016).
Lee, W.-J. et al. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Sci. Rep. 8, 1280 (2018).
Carrette, O. et al. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics 3, 1486–1494 (2003).
Brew, B. J., Dunbar, N., Pemberton, L. & Kaldor, J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J. Infect. Dis. 174, 294–298 (1996).
Starkey, H. D. V. et al. Neuroglial expression of the MHCI pathway and PirB receptor is upregulated in the hippocampus with advanced aging. J. Mol. Neurosci. 48, 111–126 (2012).
Huh, G. S. et al. Functional requirement for class I MHC in CNS development and plasticity. Science 290, 2155–2159 (2000).
Lin, K. et al. MHC class I H2-Kb negatively regulates neural progenitor cell proliferation by inhibiting FGFR signaling. PLoS Biol. 19, e3001311 (2021).
Bogeska, R. et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 29, 1273–1284 (2022).
Ho, T. T. et al. Aged hematopoietic stem cells are refractory to bloodborne systemic rejuvenation interventions. J. Exp. Med. 218, e20210223 (2021).
Jin, Z.-G. et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24, 1186–1191 (2004).
Tchalla, A. E. et al. Elevated soluble vascular cell adhesion molecule-1 is associated with cerebrovascular resistance and cognitive function. J. Gerontol. A Biol. Sci. Med. Sci. 72, 560–566 (2017).
Zuliani, G. et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J. Neurol. Sci. 272, 164–170 (2008).
Park, M. H. et al. Vascular and neurogenic rejuvenation in aging mice by modulation of ASM. Neuron 100, 167–182 (2018).
Biessels, G. J. & Despa, F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 14, 591–604 (2018).
Bocarsly, M. E. et al. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl Acad. Sci. USA 112, 15731–15736 (2015).
Cifre, M., Palou, A. & Oliver, P. Cognitive impairment in metabolically-obese, normal-weight rats: identification of early biomarkers in peripheral blood mononuclear cells. Mol. Neurodegener. 13, 14 (2018).
Parikh, I. et al. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging 8, 2814–2826 (2016).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Ruckh, J. M. et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10, 96–103 (2012).
Loffredo, F. S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Salpeter, S. J. et al. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes 62, 2843–2848 (2013).
Yousefzadeh, M. J. et al. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience 42, 951–961 (2020).
Khrimian, L. et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 214, 2859–2873 (2017). This study identified osteocalcin as a bone-derived rejuvenating factor in young blood.
Mehdipour, M. et al. Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline–albumin. Aging 12, 8790–8819 (2020). This study established neutral blood exchange as a new rejuvenating intervention.
Mehdipour, M. et al. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. Geroscience 43, 1–18 (2020).
Middeldorp, J. et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 73, 1325–1333 (2016).
Boada, M. et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: primary results of the AMBAR Study. Alzheimer’s Dement. 16, 1412–1425 (2020).
Speisman, R. B., Kumar, A., Rani, A., Foster, T. C. & Ormerod, B. K. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav. Immun. 28, 25–43 (2013).
Rhyu, I. J. et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 167, 1239–1248 (2010).
Erickson, K. I. et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl Acad. Sci. USA 108, 3017–3022 (2011).
Ding, Y.-H. et al. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc. Res. 3, 15–23 (2006).
Casaletto, K. et al. Late‐life physical activity relates to brain tissue synaptic integrity markers in older adults. Alzheimers Dement. 18, 2023–2035 (2022).
Miguel, Z. D. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Neeper, S. A., Góauctemez-Pinilla, F., Choi, J. & Cotman, C. Exercise and brain neurotrophins. Nature 373, 109 (1995).
Wahl, D. et al. Comparing the effects of low-protein and high-carbohydrate diets and caloric restriction on brain aging in mice. Cell Rep. 25, 2234–2243 (2018).
Dal-Pan, A. et al. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS ONE 6, e16581 (2011).
Leclerc, E. et al. The effect of caloric restriction on working memory in healthy non-obese adults. CNS Spectr. 25, 2–8 (2020).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009). This seminal study demonstrates the benefits that caloric restriction has on lifespan and age-associated brain atrophy in rhesus macaques.
Lin, A.-L., Zhang, W., Gao, X. & Watts, L. Caloric restriction increases ketone bodies metabolism and preserves blood flow in aging brain. Neurobiol. Aging 36, 2296–2303 (2015).
Kaur, M., Sharma, S. & Kaur, G. Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology 9, 441–454 (2008).
Lee, J., Seroogy, K. B. & Mattson, M. P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 80, 539–547 (2002).
Morgan, T. E. et al. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 89, 687–699 (1999).
Ferreira-Marques, M. et al. Caloric restriction stimulates autophagy in rat cortical neurons through neuropeptide Y and ghrelin receptors activation. Aging 8, 1470–1484 (2016).
McPherron, A. C., Lawler, A. M. & Lee, S.-J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 22, 260–264 (1999).
Egerman, M. A. et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 22, 164–174 (2015).
Ozek, C., Krolewski, R. C., Buchanan, S. M. & Rubin, L. L. Growth differentiation factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci. Rep. 8, 17293 (2018).
Mayweather, B. A., Buchanan, S. M. & Rubin, L. L. GDF11 expressed in the adult brain negatively regulates hippocampal neurogenesis. Mol. Brain 14, 134 (2021).
Oury, F. et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 155, 228–241 (2013).
Castellano, J. M. et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 544, 488–492 (2017).
McLay, R. N., Kimura, M., Banks, W. A. & Kastin, A. J. Granulocyte–macrophage colony-stimulating factor crosses the blood–brain and blood–spinal cord barriers. Brain 120, 2083–2091 (1997).
Boyd, T. D. et al. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers Dis. 21, 507–518 (2010).
Gan, K. J. & Südhof, T. C. Specific factors in blood from young but not old mice directly promote synapse formation and NMDA-receptor recruitment. Proc. Natl Acad. Sci. USA 116, 12524–12533 (2019).
Yang, J. et al. Association of accelerated long-term forgetting and senescence-related blood-borne factors in asymptomatic individuals from families with autosomal dominant Alzheimer’s disease. Alzheimers Res. Ther. 13, 107 (2021).
Kurosu, H. et al. Suppression of aging in mice by the hormone klotho. Science 309, 1829–1833 (2005).
Leon, J. et al. Peripheral elevation of a klotho fragment enhances brain function and resilience in young, aging, and α-synuclein transgenic mice. Cell Rep. 20, 1360–1371 (2017). Systemic administration of the longevity factor α-klotho enhances cognitive function in aged mice and models of neurodegeneration.
Hu, M. C. et al. Renal production, uptake, and handling of circulating αklotho. J. Am. Soc. Nephrol. 27, 79–90 (2016).
Zhu, L. et al. Klotho controls the brain–immune system interface in the choroid plexus. Proc. Natl Acad. Sci. USA 115, E11388–E11396 (2018).
Chow, L. S. et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 18, 273–289 (2022).
Schwarz, A. J., Brasel, J. A., Hintz, R. L., Mohan, S. & Cooper, D. M. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J. Clin. Endocrinol. Metab. 81, 3492–3497 (1996).
Reinhardt, R. R. & Bondy, C. A. Insulin-like growth factors cross the blood–brain barrier. Endocrinology 135, 1753–1761 (1994).
Carro, E., Nuñez, A., Busiguina, S. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 20, 2926–2933 (2000).
Trejo, J. L., Carro, E. & Torres-Aleman, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634 (2001).
Markowska, A. L., Mooney, M. & Sonntag, W. E. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience 87, 559–569 (1998).
Ding, Q., Vaynman, S., Akhavan, M., Ying, Z. & Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140, 823–833 (2006).
Li, X. et al. Cap‐independent translation of GPLD1 enhances markers of brain health in long‐lived mutant and drug‐treated mice. Aging Cell 21, e13685 (2022).
Leiter, O. et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab. 34, 408–423 (2022).
Pohlkamp, T., Wasser, C. R. & Herz, J. Functional roles of the interaction of APP and lipoprotein receptors. Front. Mol. Neurosci. 10, 54 (2017).
Schwarz, M. et al. Potential protective role of apoprotein J (clusterin) in atherogenesis: binding to enzymatically modified low-density lipoprotein reduces fatty acid-mediated cytotoxicity. Thromb. Haemost. 100, 110–118 (2008).
Islam, M. R. et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 3, 1058–1070 (2021). The systemic myokine irisin reduces neuroinflammation and enhances cognitive function in models of AD pathology.
Boström, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Lourenco, M. V. et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 25, 165–175 (2019).
Fabel, K. et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812 (2003).
Hayek, L. E. et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 39, 2369–2382 (2019).
Moon, H. Y. et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 24, 332–340 (2016).
Kestin, A. S. et al. Effect of strenuous exercise on platelet activation state and reactivity. Circulation 88, 1502–1511 (2018).
Leiter, O. et al. Exercise-induced activated platelets increase adult hippocampal precursor proliferation and promote neuronal differentiation. Stem Cell Rep. 12, 667–679 (2019).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Reger, M. A. et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 25, 311–314 (2004).
Murray, A. J. et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 30, 4021–4032 (2016).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557 (2017).
Kashiwaya, Y. et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 34, 1530–1539 (2013).
Dani, N. et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell 184, 3056–3074 (2021).
Baruch, K. et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014).
Baird, G. S. et al. Age-dependent changes in the cerebrospinal fluid proteome by slow off-rate modified aptamer array. Am. J. Pathol. 180, 446–456 (2012).
Silva-Vargas, V., Maldonado-Soto, A. R., Mizrak, D., Codega, P. & Doetsch, F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19, 643–652 (2016).
Iram, T. et al. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature 605, 509–515 (2022).
Acknowledgements
This work was supported by the Simons Foundation (S.A.V.), the Larry L. Hillblom Foundation (G.B.) and the National Institute on Aging (AG064823 (A.B.S.), AG077770 (S.A.V.), AG067740 (S.A.V.)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Fernanda De Felice and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bieri, G., Schroer, A.B. & Villeda, S.A. Blood-to-brain communication in aging and rejuvenation. Nat Neurosci 26, 379–393 (2023). https://doi.org/10.1038/s41593-022-01238-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01238-8
This article is cited by
-
Quercetin Ameliorates Cognitive Impairment in Depression by Targeting HSP90 to Inhibit NLRP3 Inflammasome Activation
Molecular Neurobiology (2024)
-
Healthy blood, healthy brain: a window into understanding and treating neurodegenerative diseases
Journal of Neurology (2024)
-
Platelet factors attenuate inflammation and rescue cognition in ageing
Nature (2023)