Abstract
Anxiety-like behaviors in mice include social avoidance and avoidance of bright spaces. Whether these features are distinctly regulated is unclear. We demonstrate that in mice, social and anxiogenic stimuli, respectively, increase and decrease serotonin (5-HT) levels in basal amygdala (BA). In dorsal raphe nucleus (DRN), 5-HT∩vGluT3 neurons projecting to BA parvalbumin (DRN5-HT∩vGluT3-BAPV) and pyramidal (DRN5-HT∩vGluT3-BAPyr) neurons have distinct intrinsic properties and gene expression and respond to anxiogenic and social stimuli, respectively. Activation of DRN5-HT∩vGluT3→BAPV inhibits 5-HT release via GABAB receptors on serotonergic terminals in BA, inducing social avoidance and avoidance of bright spaces. Activation of DRN5-HT∩vGluT3→BA neurons inhibits two subsets of BAPyr neurons via 5-HT1A receptors (HTR1A) and 5-HT1B receptors (HTR1B). Pharmacological inhibition of HTR1A and HTR1B in BA induces avoidance of bright spaces and social avoidance, respectively. These findings highlight the functional significance of heterogenic inputs from DRN to BA subpopulations in the regulation of separate anxiety-related behaviors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA sequencing raw data have been deposited in the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) with the access number GSE214895. The data that support this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Craske, M. G. & Stein, M. B. Anxiety. Lancet 388, 3048–3059 (2016).
Tovote, P., Fadok, J. P. & Luthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015).
Griebel, G. & Holmes, A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 12, 667–687 (2013).
Calhoon, G. G. & Tye, K. M. Resolving the neural circuits of anxiety. Nat. Neurosci. 18, 1394–1404 (2015).
Tyrer, P. & Baldwin, D. Generalised anxiety disorder. Lancet 368, 2156–2166 (2006).
Adhikari, A. et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527, 179–185 (2015).
Barchas, J. D., Akil, H., Elliott, G. R., Holman, R. B. & Watson, S. J. Behavioral neurochemistry: neuroregulators and behavioral states. Science 200, 964 (1978).
Dayan, P. & Huys, Q. J. Serotonin in affective control. Annu. Rev. Neurosci. 32, 95–126 (2009).
Stein, M. B. & Andrews, A. M. Serotonin states and social anxiety. JAMA Psychiatry 72, 845–847 (2015).
Holmes, A., Murphy, D. L. & Crawley, J. N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol. Psychiatry 54, 953–959 (2003).
Ji, G. et al. 5-HT2C receptor knockdown in the amygdala inhibits neuropathic-pain-related plasticity and behaviors. J. Neurosci. 37, 1378–1393 (2017).
Johnson, P. L. et al. Pharmacological depletion of serotonin in the basolateral amygdala complex reduces anxiety and disrupts fear conditioning. Pharmacol. Biochem. Behav. 138, 174–179 (2015).
Bocchio, M. et al. Increased serotonin transporter expression reduces fear and recruitment of parvalbumin interneurons of the amygdala. Neuropsychopharmacology 40, 3015–3026 (2015).
Gross, C. et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416, 396–400 (2002).
Marcinkiewcz, C. A. et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101 (2016).
Wu, X., Morishita, W., Beier, K. T., Heifets, B. D. & Malenka, R. C. 5-HT modulation of a medial septal circuit tunes social memory stability. Nature 599, 96–101 (2021).
Dölen, G., Darvishzadeh, A., Huang, K. W. & Malenka, R. C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179 (2013).
Walsh, J.J. et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560, 589–594 (2018).
Nardou, R. et al. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 569, 116–120 (2019).
Sengupta, A. & Holmes, A. A discrete dorsal raphe to basal amygdala 5-HT circuit calibrates aversive memory. Neuron 103, 489–505 (2019).
Li, Y. et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat. Commun. 7, 10503 (2016).
Cohen, J. Y., Amoroso, M. W. & Uchida, N. Serotonergic neurons signal reward and punishment on multiple timescales. eLife 4, e06346 (2015).
Jacobs, B. L. & Azmitia, E. C. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229 (1992).
Ren, J. et al. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175, 472–487 (2018).
Okaty, B. W., Commons, K. G. & Dymecki, S. M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 20, 397–424 (2019).
Ren, J. et al. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. eLife 8, e49424 (2019).
Asan, E., Steinke, M. & Lesch, K. P. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem. Cell Biol. 139, 785–813 (2013).
Capogna, M. GABAergic cell type diversity in the basolateral amygdala. Curr. Opin. Neurobiol. 26, 110–116 (2014).
Wolff, S. B. et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 509, 453–458 (2014).
Fustiñana, M.S., Eichlisberger, T., Bouwmeester, T., Bitterman, Y. & Lüthi, A. State-dependent encoding of exploratory behaviour in the amygdala. Nature 592, 267–271 (2021).
Beyeler, A. et al. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron 90, 348–361 (2016).
Beyeler, A. et al. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 22, 905–918 (2018).
Burgos-Robles, A. et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 20, 824–835 (2017).
Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19, 1636–1646 (2016).
Gründemann, J. et al. Amygdala ensembles encode behavioral states. Science 364, v8736 (2019).
Namburi, P. et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015).
Shen, C. J. et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 25, 337–349 (2019).
Janak, P. H. & Tye, K. M. From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015).
Fu, J. et al. Whole-brain map of long-range monosynaptic inputs to different cell types in the amygdala of the mouse. Neurosci. Bull. 36, 1381–1394 (2020).
Zhou, W. et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat. Neurosci. 22, 1945 (2019).
Barkus, C. et al. Variation in serotonin transporter expression modulates fear-evoked hemodynamic responses and theta-frequency neuronal oscillations in the amygdala. Biol. Psychiatry 75, 901–908 (2014).
Yamamoto, R., Hatano, N., Sugai, T. & Kato, N. Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT(2C) receptor. Neuropharmacology 82, 49–58 (2014).
Lister, R. G. & Hilakivi, L. A. The effects of novelty, isolation, light and ethanol on the social behavior of mice. Psychopharmacology 96, 181–187 (1988).
Sah, P., Faber, E. S., Lopez, D. A. M. & Power, J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 83, 803–834 (2003).
Bocchio, M., McHugh, S. B., Bannerman, D. M., Sharp, T. & Capogna, M. Serotonin, amygdala and fear: assembling the puzzle. Front. Neural Circuits 10, 24 (2016).
Tao, R., Ma, Z. & Auerbach, S. B. Differential regulation of 5-hydroxytryptamine release by GABAA and GABAB receptors in midbrain raphe nuclei and forebrain of rats. Br. J. Pharmacol. 119, 1375–1384 (1996).
Lecca, S. et al. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat. Med. 22, 254–261 (2016).
LeDoux, J. The amygdala. Curr. Biol. 17, R868–R874 (2007).
Calizo, L. H. et al. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61, 524–543 (2011).
Gaspar, P. & Lillesaar, C. Probing the diversity of serotonin neurons. Phil. Trans. R. Soc. B 367, 2382–2394 (2012).
Okaty, B. W. et al. Multi-scale molecular deconstruction of the serotonin neuron system. Neuron 88, 774–791 (2015).
Zou, W. J. et al. A discrete serotonergic circuit regulates vulnerability to social stress. Nat. Commun. 11, 4218 (2020).
Brown, J. T., Booth, C. A. & Randall, A. D. Synaptic activation of mGluR1 generates persistent depression of a fast after-depolarizing potential in CA3 pyramidal neurons. Eur. J. Neurosci. 33, 879–889 (2011).
Liu, Z. et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81, 1360–1374 (2014).
Richardson-Jones, J. W. et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65, 40–52 (2010).
Richardson-Jones, J. W. et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J. Neurosci. 31, 6008–6018 (2011).
Wan, J. et al. A genetically encoded sensor for measuring serotonin dynamics. Nat. Neurosci. 24, 746–752 (2021).
Sengupta, A., Bocchio, M., Bannerman, D. M., Sharp, T. & Capogna, M. Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J. Neurosci. 37, 1785–1796 (2017).
Bocchio, M., Nabavi, S. & Capogna, M. Synaptic plasticity, engrams, and network oscillations in amygdala circuits for storage and retrieval of emotional memories. Neuron 94, 731–743 (2017).
Bazelot, M. et al. Hippocampal theta input to the amygdala shapes feedforward inhibition to gate heterosynaptic plasticity. Neuron 87, 1290–1303 (2015).
Guo, J. D., O’Flaherty, B. M. & Rainnie, D. G. Serotonin gating of cortical and thalamic glutamate inputs onto principal neurons of the basolateral amygdala. Neuropharmacology 126, 224–232 (2017).
Nautiyal, K. M. et al. A lack of serotonin 1B autoreceptors results in decreased anxiety and depression-related behaviors. Neuropsychopharmacology 41, 2941–2950 (2016).
van Vliet, I. M., den Boer, J. A., Westenberg, H. G. & Pian, K. L. Clinical effects of buspirone in social phobia: a double-blind placebo-controlled study. J. Clin. Psychiatry 58, 164–168 (1997).
Albert, P. R. & Vahid-Ansari, F. The 5-HT1A receptor: signaling to behavior. Biochimie 161, 34–45 (2019).
Murrough, J. W. & Neumeister, A. The serotonin 1B receptor: a new target for depression therapeutics? Biol. Psychiatry 69, 714–715 (2011).
Da, C. S. et al. Men with high serotonin 1B receptor binding respond to provocations with heightened amygdala reactivity. Neuroimage 166, 79–85 (2018).
Nautiyal, K. M. et al. Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 86, 813–826 (2015).
Hjorth, O. R. et al. Expression and co-expression of serotonin and dopamine transporters in social anxiety disorder: a multitracer positron emission tomography study. Mol. Psychiatry 26, 3970–3979 (2021).
Felix-Ortiz, A. C. et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013).
Felix-Ortiz, A. C. & Tye, K. M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 34, 586–595 (2014).
Pi, G. et al. Posterior basolateral amygdala to ventral hippocampal CA1 drives approach behaviour to exert an anxiolytic effect. Nat. Commun. 11, 183 (2020).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Gothard, K. M. Multidimensional processing in the amygdala. Nat. Rev. Neurosci. 21, 565–575 (2020).
Kyriazi, P., Headley, D. B. & Pare, D. Multi-dimensional coding by basolateral amygdala. Neuron 99, 1315–1328 (2018).
Blume, S. R. et al. Sex- and estrus-dependent differences in rat basolateral amygdala. J. Neurosci. 37, 10567–10586 (2017).
Acknowledgements
We thank S. M. Duan (Zhejiang University, China), H. L. Hu (Zhejiang University, China), Z. M. Lu (Zhejiang University, China), T. M. Gao (Southern Medical University, China), Y. H. Cui (Zhejiang University, China), G. P. Feng (Massachusetts Institute of Technology, USA) and all members of X.-M. Li’s Lab for discussions and suggestions on experimental design and manuscript preparation. We thank T. F. Yuan (Shanghai Jiao Tong University, China) for providing the FSCV technology. We thank Z. J. Huang (Cold Spring Harbor Laboratory, USA), H. Miao (Fudan University, China), X. H. Zhang (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China), Y. Rao (Capital Medical University, China) and M. M. Luo (National Institute of Biological Sciences, Beijing, China) for providing transgenic mouse lines. We thank the Chuanqi Research and Development Centre of Zhejiang University and the Core Facilities of the Zhejiang University Institute of Neuroscience for technical support. This study was funded by the major programme of the National Natural Science Foundation of China (82090031 and 82090030), Science and Technology Innovation 2030—major project of Brain Science and Brain-Like Research (2021ZD0202702 and 2021ZD0202700), General Programme of the NSFC (31871070), Key-Area Research and Development Program of Guangdong Province (2018B030334001 and 2019B030335001), Key R&D Program of Zhejiang Province (2020C03009), the Fundamental Research Funds for the Central Universities (2021FZZX001-37), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-057) and Innovative and Entrepreneur Team of Zhejiang for the year 2020 biomarker-driven basic and translational research on major brain diseases (2020R01001) to X.-M.L. and Young Scientist Program of the NSFC (82001454) to H.H.
Author information
Authors and Affiliations
Contributions
X.-D.Y. and X.-M.L. conceptualized and designed the experiment. X.-D.Y. performed the experiments and data analyses, including fiber photometry, FSCV, optogenetic, RNA-seq and ex vivo electrophysiological, behavioral, and immunohistochemical experiments. Y.Z., J.-Y.F. and D.Z. helped with the RNA scope analyses. F.D. and J.-X.W. helped with the GRAB5-HT2h signal recordings. Q.-X.S. helped with data analysis and stereotaxic surgeries. C.-J.S. and S.-Z.X. helped with stereotaxic surgeries. W.-K.G. helped with the FSCV recordings. H.Q.H., H.-Y.L., J.J. and Y.-L.L. helped with data analysis. The manuscript was written by X.-D.Y. and X.-M.L. X.-M.L. supervised all phases of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Anna Beyeler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 High Ambient Illumination Induces Anxiety-related Behaviours.
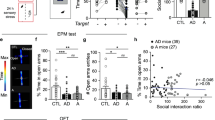
Representative traces and statistics (mean ± s.e.m) of time in open arm (F(2, 57) = 11.13, P < 0.0001; 20–300 Lux, t(57) = 3.563, **P = 0.0015; 20–600 Lux, t(57) = 4.459, ***P = 0.0001; 300–600 Lux, t(57) = 0.8959, P = 0.3741) of EPM (a), time in centre (F(2, 57) = 4.716, P = 0.0127; 20–300 Lux, t(57) = 2.760, *P = 0.0231; 20–600 Lux, t(57) = 2.547, *P = 0.0270; 300–600 Lux, t(57) = 0.2125, P = 0.8324) and total distance (F(2, 57) = 0.6638, P = 0.5188) in OFT (b), latency to feed (F(2, 57) = 22.45, P < 0.0001; 20–300 Lux, t(57) = 0.1996, P = 0.8425; 20–600 Lux, t(57) = 5.900, ****P < 0.0001; 300–600 Lux, t(57) = 5.701, ****P < 0.0001) and total food consumed (F(2, 57) = 0.1877, P = 0.8294) in NSF (c), and interaction time (F(2, 57) = 26.33, P < 0.0001; 20–300 Lux, t(57) = 3.247, **P = 0.002; 20–600 Lux, t(57) = 7.244, ****P < 0.0001; 300–600 Lux, t(57) = 0.3997, ***P = 0.0004) in social interaction test (d) (two-sided RM one-way ANOVA followed by Holm–Sidak correction), sniffing time (F(5, 114) = 18.46, P < 0.0001; S1, 20–300 Lux, t(228) = 4.591, ****P < 0.0001, 20–600 Lux, t(228) = 8.918, ****P < 0.0001, 300–600 Lux, t(228) = 4.327, ***P = 0.0002; S2, 20–300 Lux, t(228) = 1.347, P = 0.5467, 20–600 Lux, t(228) = 5.765, ****P < 0.0001, 300–600 Lux, t(228) =4.419, ***P = 0.0001) and social index (F(2, 57) = 1.358, P = 0.2653; F(2, 57) illumination = 25.31, P < 0.0001; sociability, 20–300 Lux, t(114) = 2.632, **P = 0.0097; 20–600 Lux, t(114) = 5.568, ****P < 0.0001; 300–600 Lux, t(114) = 2.936, **P = 0.0080; social novelty, 20–300 Lux, t(114) = 0.4221, P = 0.6738; 20–600 Lux, t(114) = 3.393, ****P < 0.0001; 300–600 Lux, t(114) = 2.971, **P = 0.0072) in three-chamber test (e; two-sided RM two-way ANOVA followed by Holm–Sidak correction) (n = 20 mice).
Extended Data Fig. 2 Dynamics of Extracellular 5-HT Level in BA of Female and Male Mice under Social and Anxiogenic Stimuli.
(a, b) Average traces (a, mean ± 95% CI; dash line: start of social interaction) and statistics (b, GFP, n = 5 mice; GRAB5-HT2h, n = 6 mice; two-sided RM two-way ANOVA followed by Holm–Sidak correction, F(1, 9) = 62.69, P < 0.0001; GRAB5-HT2h Baseline versus GRAB5-HT2h Social, ****P < 0.0001; GFP Social versus GRAB5-HT2h Social, ****P < 0.0001) of GFP and GRAB5-HT2h fluorescence under social stimulus in female mice. (c, d) Average traces (c, mean ± 95% CI; dash line: start of ~300 Lux stimulus) and statistics (d, GFP, n = 5 mice; GRAB5-HT2h, n = 6 mice; two-sided RM two-way ANOVA followed by Holm–Sidak correction, F(1, 9) = 23.98, P = 0.0009; GRAB5-HT2h 20 versus 300 Lux, ****P < 0.0001; 300 Lux, GFP versus GRAB5-HT2h, ****P < 0.0001) of GFP and GRAB5-HT2h fluorescence under ~300 Lux stimulus in female mice. (e, f) Average traces (e, mean ± 95% CI; dash line: start of social interaction) and statistics (f, mean ± SEM; n = 6 female and 7 male mice; two-sided unpaired t test; t(11) = 0.2916, P = 0.7760) of GRAB5-HT2h fluorescence in male and female mice under social stimulus. (g, h) Average traces (g, mean ± 95% CI; dash line: start of ~300 Lux stimulus) and statistics (h, mean ± SEM; n = 6 female and 6 male mice; two-sided unpaired t test; t(10) = 0.5323, P = 0.6062) of GRAB5-HT2h fluorescence in male and female mice under ~300 Lux stimulus.
Extended Data Fig. 3 FSCV Recordings of the Extracellular 5-HT Dynamics in BA.
(a-d) Representative pseudo colour plots (a), voltammograms (b), traces (c) and statistics (d, n = 9 events from 3 mice; two-sided paired t test; t(8) = 7.512, ****P < 0.0001) of changes of 5-HT under social stimulus, triangles in a represent time point of voltammograms in b (e-g) Representative pseudo colour plots (e) and voltammograms (f), traces (g) and statistics (h, n = 13 events from 3 mice; two-sided paired t test; t(12) = 4.495, ***P = 0.0007) of changes in 5-HT under ~300 Lux stimulus, triangles in e represent time point of voltammograms in f (i) Voltage applied to carbon fiber electrodes during FSCV recording of 5-HT. (j-m) Schematic of virus injection (j, l) and 5-HT cyclic voltammograms (k, m, background subtracted) after opto-stimulation of DRN5-HT neurons.
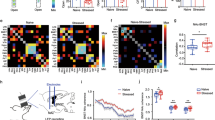
Extended Data Fig. 4 Differential Expressions of Genes in DRN-BAPyr and DRN-BAPV Neurons.
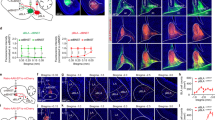
(a-c) FPKM of genes related to synthesis and secretion of neurotransmitters (a), ion channels (b) and neurotransmitter release (c) in DRN-BAPyr and DRN-BAPV neurons.
Extended Data Fig. 5 New Environment Exposure Induces Anxiety-like Behaviours and Increases cFos Expression in DRN-BAPV but not DRN-BAPyr Neurons.
(a) Schematics of new environment pre-exposure. (b, c) Representative traces and statistics (mean ± SEM; n = 13 mice; two-sided unpaired t test) of time in open arm (t(24) = 4.616, ***P = 0.0001) in EPM (b) and time in centre (t(24) = 2.303, *P = 0.0302) and total distance (t(24) = 0.1184, P = 0.9067) in OFT (c) of home cage (HC) or non-home cage (NHC) pre-exposure mice. (d, e) Representative images (d) and statistics (e, mean ± SEM; n = 5, 6, 6, and 6 mice; two-sided RM one-way ANOVA, F(3, 19) = 67.69, P < 0.0001; HC, vGluT2 versus PV, t(19) = 4.052, **P = 0.0016; PV, NC versus NHC, t(19) = 8.32, ****P < 0.0001) of cFos expression in RV-dsRed-positive neurons of PV-ires-cre and vGluT2-ires-cre mice in DRN exposed to home cage (HC) or non-home cage (NHC), arrows represent co-expression of cFos and dsRed, triangles represent non-cFos expression in dsRed+ neurons, scale bar: 50 μm.
Extended Data Fig. 6 BAPV Neurons are Activated under Anxiogenic but not Social Stimulus.
Schematics of anxiogenic and social stimuli (a, top), representative images (a, bottom) and statistics (b, mean ± SEM; n = 6, 8, 8 and 8 mice; F(3, 26) = 10.84, P < 0.0001; two-sided RM one-way ANOVA; Non-social, 20 versus 300 Lux, t(26) = 3.543, **P = 0.0046; Social, 20 Lux versus 300 Lux, t(26) = 4.468, ***P = 0.0008) of cFos expression in BAPV neurons, arrows: co-expression of cFos and PV, triangles: non-cFos expression in BAPV neurons, scale bar: 50 μm.
Extended Data Fig. 7 Social Stimulus Decrease cFos Expression in BAPyr neurons.
Schematics of anxiogenic and social stimuli (a, top), representative images (a, bottom) and statistics (b, mean ± SEM; n = 8 mice; F(3, 28) = 23.54, P < 0.0001; two-sided RM one-way ANOVA; 20 Lux, Non-social versus Social, t(28) = 3.389, **P = 0.0063; 300 Lux, Non-social versus Social, t(28) = 5.33, ****P < 0.0001; Non-social, 20 versus 300 Lux, t(28) 4.89, ***P = 0.0001; Social, 20 versus 300 Lux, t(28) = 2.949, *P = 0.0127) of cFos expression in BAPyr neurons, arrows: co-expression of cFos and PV, triangles: non-cFos expression in BAPyr neurons, scale bar: 50 μm.
Extended Data Fig. 8 DRN5-HT Neurons Inhibit a Subset of BAPyr Neurons via HTR1A but not GABA.
(a) Schematic of virus injection and electrophysiological recordings in BAPyr neurons under 5-ms light pulse stimulation of serotonergic terminals from DRN. (b) Representative trace of BAPyr neurons using a high chloride internal solution under 5-ms light pulse in the presence of TTX + 4AP. (c) Schematic of virus injection and electrophysiological recordings in BAPyr neurons under 55-Hz light train stimulation of serotonergic terminals from DRN. (d) Proportion of neurons with (responsive) or without (non-responsive) slow oIPSCs in BAPyr neurons during 55-Hz light train. (e) Diagrams of distribution of responsive (solid circles) and non-responsive (hollow circles) BAPyr neurons. (f) Representative traces (left, shadow: opto-activation) and statistics (right; n = 9 neurons from 3 mice; two-sided RM one-way ANOVA; F(8,16) = 27.17, P < 0.0001; ACSF versus CGP54626, t(8) = 1.132, P = 0.2904; CGP54626 versus WAY100635, t(8) = 5.815, **P = 0.0012; CGP54626 versus WAY100635, t(8) = 5.495, **P = 0.0012) of oIPSCs before and after application of CGP54626 (GABAB receptor antagonist, 1 μM) and WAY100635. (g) Representative traces (left, shadow: opto-activation) statistics (right; n = 6 neurons from 4 mice; two-sided paired t test; t(5) = 1.772, P = 0.1366) of oIPSCs before and after application of NAS-181 (1 μM).
Extended Data Fig. 9 Activation of Serotonergic Terminals in BA Modulates Glutamate Transmission to HTR1A− Pyr Neurons, but not PV or HTR1A+ Neurons, via HTR1B.
(a-c) Schematic (a), representative traces (b) and statistics (c, mean ± SEM and individual data; n = 21 neurons from 4 mice; two-sided RM one-way ANOVA, F(20, 180) = 9.516, P < 0.0001) of the amplitude of oEPSCs in BAPV neurons that showed glutamatergic responses (R-BAPV neurons) in response to opto-stimulation. (d-f) Schematic (d), representative traces (e), cumulative frequency curves of inter-spike-interval (f, outside), and statistics (f, inside) of frequency (f, left; t(16) = 0.9465, P = 0.3580) and amplitude (f, right; t(16) = 0.6886, P = 0.5009) of sEPSCs in BAPV neurons that did not show glutamatergic responses (NR-BAPV neurons) before (light off) and after (light on) opto-stimulation (n = 17 neurons from 4 mice, two-sided paired t test). (g-i) Schematic (g), representative traces (h), cumulative frequency curves of inter-spike-interval (i, outside), and statistics (i, inside) of frequency (i, left; t(11) = 0.2341, NS P = 0.8192) and amplitude (i, right; t(11) = 1.166, NS P = 0.8192) of sEPSCs in HTR1A+ BAPyr neurons before (light off) and after (light on) opto-stimulation (n = 12 neurons from 4 mice, two-sided paired t test). (j-l) Schematic (j), representative traces (k), cumulative frequency curves of inter-spike-interval (l, outside), and statistics (l, inside) of frequency (t(29) = 5.419, ****P < 0.0001) and amplitude (t(29) = 0.3166, P = 0.7538) of sEPSCs in BA HTR1A− neurons before and after opto-stimulation (n = 30 neurons from 4 mice; two-sided paired t test). (m, n) Representative traces (m), cumulative frequency curves of inter-spike-interval (n, outside), and statistics (n, inside) of frequency (t(28) = 0.9280; P = 0.3612) and amplitude (t(28) = 0.6915; P = 0.4950) of sEPSCs in BA HTR1A− neurons before and after opto-train stimulation with the presence of NAS-181 (1 μM) (n = 29 neurons from 4 mice, two-sided paired t test). Shadows in f, i, l, k: opto-activation.
Extended Data Fig. 10 Pharmacological Inhibiting HTR1A in BA Induced Avoidance of Bright Spaces but not Social Avoidance, without Affecting Locomotion.
(a) Schematic paradigm of cannula implantation and drug administration. (b-f) Statistics (mean ± SEM; n = 13 mice, two-sided unpaired t test) of time in open arm (t(24) = 4.324; ***P = 0.0002) in EPM (b), time in centre (t(24) = 3.718; **P = 0.0011) in OFT (c), latency to feed (t(24) = 3.411; **P = 0.0023) in NSF (d) and interaction time (t(24) = 1.483; P = 0.1512) in social interaction test (e), sniffing time (F(3, 48) = 13.78, P < 0.0001; E versus S1, ACSF, t(48) = 11.39, ****P < 0.0001, WAY100635, t(48) = 9.254, ****P < 0.0001; S1 versus S2, ACSF, t(48) = 4.218, ****P = 0.0001, WAY100635, t(48) = 0.703, ****P < 0.0001; ACSF versus WAY100635, S1, t(96) = 2.551, *P = 0.0245, S2, t(96) = 0.3671, P = 0.7144), social index (F(1, 24)pharmacology = 0.0945, P = 0.7611; ACSF versus WAY100635, sociability, t(48) = 0.9231, P = 0.36.6, social novelty, t(48) = 1.347, P = 0.3347) in three-chamber test (f; n = 13 mice; two-sided RM two-way ANOVA) after administration of ACSF or WAY100635 in BA bilaterally in C57 mice. (g) Schematic paradigm of cannula implantation and drug administration. (h-i) Statistics (mean ± SEM) of total distance (F(2, 36) = 1.659; P = 0.2045) in OFT (h) and total food consumed (F(2, 36) = 0.196; P > 0.8229) in NSF (i) after administration of ACSF, WAY100635 and NAS-181 in BA bilaterally in C57 mice (n = 13 mice, two-sided RM one-way ANOVA).
Supplementary information
Supplementary Information
Supplementary Figs. 1–13 and Supplementary Table 1.
Supplementary Data 1
Statistical data for supplementary figures.
Source data
Source Data Fig. 2
Statistical results of sniffing time (s) in three-chamber test.
Source Data Fig. 4
Statistical results of sniffing time (s) in three-chamber test.
Source Data Fig. 5
Statistical results of vGAT fluorescence intensity.
Source Data Fig. 8
Statistical results of sniffing time (s) in three-chamber test.
Source Data Extended Data Fig. 1
Statistical results of sniffing time (s) in three-chamber test.
Source Data Extended Data Fig. 9
Statistical results of sniffing time (s) in three-chamber test.
Source Data Extended Data Fig. 10
Statistical results of sniffing time (s) in three-chamber test.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, XD., Zhu, Y., Sun, QX. et al. Distinct serotonergic pathways to the amygdala underlie separate behavioral features of anxiety. Nat Neurosci 25, 1651–1663 (2022). https://doi.org/10.1038/s41593-022-01200-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01200-8
This article is cited by
-
A molecularly defined amygdala-independent tetra-synaptic forebrain-to-hindbrain pathway for odor-driven innate fear and anxiety
Nature Neuroscience (2024)
-
Disfunction of dorsal raphe nucleus-hippocampus serotonergic-HTR3 transmission results in anxiety phenotype of Neuroplastin 65-deficient mice
Acta Pharmacologica Sinica (2024)
-
The role of bidirectional associations between depression, anxiety, and emotional exhaustion on turnover intention among nurses: a multicenter cross-sectional study in China
BMC Nursing (2023)
-
Plasticity of neuronal dynamics in the lateral habenula for cue-punishment associative learning
Molecular Psychiatry (2023)
-
Diverse Roles of Serotonergic Projections to the Basolateral Amygdala
Neuroscience Bulletin (2023)