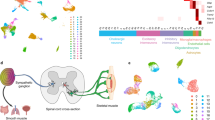
Abstract
Precisely controlled development of the somatosensory system is essential for detecting pain, itch, temperature, mechanical touch and body position. To investigate the protein-level changes that occur during somatosensory development, we performed single-cell mass cytometry on dorsal root ganglia from C57/BL6 mice of both sexes, with litter replicates collected daily from embryonic day 11.5 to postnatal day 4. Measuring nearly 3 million cells, we quantified 30 molecularly distinct somatosensory glial and 41 distinct neuronal states across all timepoints. Analysis of differentiation trajectories revealed rare cells that co-express two or more Trk receptors and over-express stem cell markers, suggesting that these neurotrophic factor receptors play a role in cell fate specification. Comparison to previous RNA-based studies identified substantial differences between many protein–mRNA pairs, demonstrating the importance of protein-level measurements to identify functional cell states. Overall, this study demonstrates that mass cytometry is a high-throughput, scalable platform to rapidly phenotype somatosensory tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Additiojnal information and requests for resources and reagents should be directed to and will be fulfilled by E.R.Z. (ezunder@virginia.edu). The unprocessed single-cell mass cytometry datasets are accessible at FlowRepository (https://flowrepository.org/) (ID: FR-FCM-Z4S9). The debarcoded sample .FCS files and clean-up gates used for pre-processing are available at Cytobank (https://community.cytobank.org/cytobank/experiments/102249). Pre-processed and annotated scRNA-seq data from Sharma et al.19 are available at https://kleintools.hms.harvard.edu/tools/springViewer_1_6_dev.html?datasets/Sharma2019/all. The scRNA-seq dataset (External Resource Table 1) from Usoskin et al.2 is available in the additional supporting data for the manuscript (http://linnarssonlab.org/drg/)2.
Code availability
The code used to perform analysis of mass cytometry data was adapted from standard R and Python packages, including UMAP, LeidenAlg and URD. The code is available on GitHub at https://github.com/zunderlab/Keeler-et-al.-DRG-Development-Manuscript. More detailed information is available upon reasonable request.
References
Lallemend, F. & Ernfors, P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 35, 373–381 (2012).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Li, C.-L. et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 26, 83–102 (2016).
Li, C., Wang, S., Chen, Y. & Zhang, X. Somatosensory neuron typing with high-coverage single-cell RNA sequencing and functional analysis. Neurosci. Bull. 34, 200–207 (2018).
Emery, E. C. & Ernfors, P. Dorsal root ganglion neuron types and their functional specialization. in The Oxford Handbook of the Neurobiology of Pain (ed. Wood, J. N.) (Oxford University Press, 2018).
Orefice, L. L. et al. Peripheral mechanosensory neuron dysfunction underlies tactile and behavioral deficits in mouse models of asds. Cell 166, 299–313 (2016).
Orefice, L. L. et al. Targeting peripheral somatosensory neurons to improve tactile-related phenotypes in ASD models. Cell 178, 867–886 (2019).
Özkaya, A. K. et al. A case of congenital insensitivity to pain with anhidrosis. Turk. Pediatr. Ars. 49, 177–179 (2014).
Darmanis, S. et al. A survey of human brain transcriptome diversity at the single cell level. Proc. Natl Acad. Sci. USA 112, 7285–7290 (2015).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Tasic, B. et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 19, 335–346 (2016).
Habib, N. et al. Div-Seq: single-nucleus RNA-seq reveals dynamics of rare adult newborn neurons. Science 353, 925–928 (2016).
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014 (2018).
Chiu, I. M. et al. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 3, e04660 (2014).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Finno, C. J. et al. Single-cell RNA-seq reveals profound alterations in mechanosensitive dorsal root ganglion neurons with vitamin E deficiency. iScience 21, 720–735 (2019).
Hockley, J. R. F. et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68, 633–644 (2019).
Faure, L. et al. Single cell RNA sequencing identifies early diversity of sensory neurons forming via bi-potential intermediates. Nat. Commun. 11, 4175 (2020).
Sharma, N. et al. The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398 (2020).
Kupari, J. et al. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat. Commun. 12, 1510 (2021).
Wang, K. et al. Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain. Cell Res. 31, 904–918 (2021).
Wu, H. et al. Distinct subtypes of proprioceptive dorsal root ganglion neurons regulate adaptive proprioception in mice. Nat. Commun. 12, 1026 (2021).
Nguyen, M. Q., von Buchholtz, L. J., Reker, A. N., Ryba, N. J. & Davidson, S. Single-nucleus transcriptomic analysis of human dorsal root ganglion neurons. eLife 10, e71752 (2021).
Bandura, D. R. et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81, 6813–6822 (2009).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Zunder, E. R., Lujan, E., Goltsev, Y., Wernig, M. & Nolan, G. P. A continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell 16, 323–337 (2015).
Behbehani, G. K., Bendall, S. C., Clutter, M. R., Fantl, W. J. & Nolan, G. P. Single-cell mass cytometry adapted to measurements of the cell cycle. Cytom. A 81, 552–566 (2012).
Fienberg, H. G., Simonds, E. F., Fantl, W. J., Nolan, G. P. & Bodenmiller, B. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytom. A 81, 467–475 (2012).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380–395 (2018).
Friebel, E. et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell 181, 1626–1642 (2020).
Ajami, B. et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551 (2018).
Ornatsky, O. I. et al. Study of cell antigens and intracellular DNA by identification of element-containing labels and metallointercalators using inductively coupled plasma mass spectrometry. Anal. Chem. 80, 2539–2547 (2008).
Wheeler, M. A. et al. TNF-α/TNFR1 signaling is required for the development and function of primary nociceptors. Neuron 82, 587–602 (2014).
Zunder, E. R. et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc. 10, 316–333 (2015).
Finck, R. et al. Normalization of mass cytometry data with bead standards. Cytom. A 83, 483–494 (2013).
Fread, K. I., Strickland, W. D., Nolan, G. P. & Zunder, E. R. An updated debarcoding tool for mass cytometry with cell type-specific and cell sample-specific stringency adjustment. Pac. Symp. Biocomput. 22, 588–598 (2017).
Schuyler, R. P. et al. Minimizing batch effects in mass cytometry data. Front. Immunol. 10, 2367 (2019).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at https://arxiv.org/abs/1802.03426 (2018).
Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018).
Wu, H.-H. et al. Glial precursors clear sensory neuron corpses during development via Jedi-1, an engulfment receptor. Nat. Neurosci. 12, 1534–1541 (2009).
Crowley, C. et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001–1011 (1994).
Fariñas, I., Yoshida, C. K., Backus, C. & Reichardt, L. F. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron 17, 1065–1078 (1996).
White, F. A. et al. Synchronous onset of NGF and TrkA survival dependence in developing dorsal root ganglia. J. Neurosci. 16, 4662–4672 (1996).
White, F. A., Keller-Peck, C. R., Knudson, C. M., Korsmeyer, S. J. & Snider, W. D. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci. 18, 1428–1439 (1998).
Patel, T. D., Jackman, A., Rice, F. L., Kucera, J. & Snider, W. D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25, 345–357 (2000).
Cheng, I., Jin, L., Rose, L. C. & Deppmann, C. D. Temporally restricted death and the role of p75NTR as a survival receptor in the developing sensory nervous system. Dev. Neurobiol. 78, 701–717 (2018).
Farrell, J. A. et al. Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science 360, eaar3131 (2018).
Britsch, S. et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66–78 (2001).
Fariñas, I., Cano-Jaimez, M., Bellmunt, E. & Soriano, M. Regulation of neurogenesis by neurotrophins in developing spinal sensory ganglia. Brain Res. Bull. 57, 809–816 (2002).
Maro, G. S. et al. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat. Neurosci. 7, 930–938 (2004).
Taylor, M. K., Yeager, K. & Morrison, S. J. Physiological Notch signaling promotes gliogenesis in the developing peripheral and central nervous systems. Development 134, 2435–2447 (2007).
Guo, W. et al. Developmental expression of P2X5 receptors in the mouse prenatal central and peripheral nervous systems. Purinergic Signal. 9, 239–248 (2013).
Levin, E., Andreadaki, A., Gobrecht, P., Bosse, F. & Fischer, D. Nociceptive DRG neurons express muscle lim protein upon axonal injury. Sci. Rep. 7, 643 (2017).
Avraham, O. et al. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 11, 4891 (2020).
Ferrari, L. F., Khomula, E. V., Araldi, D. & Levine, J. D. CD44 signaling mediates high molecular weight hyaluronan-induced antihyperalgesia. J. Neurosci. 38, 308–321 (2018).
Lee, K. F. et al. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69, 737–749 (1992).
Fan, G., Jaenisch, R. & Kucera, J. A role for p75 receptor in neurotrophin-3 functioning during the development of limb proprioception. Neuroscience 90, 259–268 (1999).
Murray, S. S., Bartlett, P. F. & Cheema, S. S. Differential loss of spinal sensory but not motor neurons in the p75NTR knockout mouse. Neurosci. Lett. 267, 45–48 (1999).
Chen, Z. et al. p75 is required for the establishment of postnatal sensory neuron diversity by potentiating ret signaling. Cell Rep. 21, 707–720 (2017).
Ko, M. E. et al. FLOW-MAP: a graph-based, force-directed layout algorithm for trajectory mapping in single-cell time course datasets. Nat. Protoc. 15, 398–420 (2020).
da Silva, R. P. & Gordon, S. Phagocytosis stimulates alternative glycosylation of macrosialin (mouse CD68), a macrophage-specific endosomal protein. Biochem. J. 338, 687–694 (1999).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Angelo, M. et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442 (2014).
Luo, W. et al. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron 54, 739–754 (2007).
Kuruvilla, R. et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118, 243–255 (2004).
Liu, Y., Beyer, A. & Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550 (2016).
Reimegård, J. et al. A combined approach for single-cell mRNA and intracellular protein expression analysis. Commun. Biol. 4, 624 (2021).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Kurtz, A. et al. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 120, 2637–2649 (1994).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Bastian, M., Heymann, S. & Jacomy, M. Gephi: an open source software for exploring and manipulating networks. Third International AAAI Conference on Weblogs and Social Media. 361–362 https://ojs.aaai.org/index.php/ICWSM/article/view/13937 (2009).
Jacomy, M., Venturini, T., Heymann, S. & Bastian, M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE 9, e98679 (2014).
Acknowledgements
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number F32NS103770 to A.B.K. and R01NS111220 to E.R.Z. and C.D.D. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Further support was provided by the 3 Cavaliers Pilot research program (to C.D.D. and E.R.Z.). We thank N. Sharma and D. Ginty for consultation about data formatting and analysis steps from their scRNA-seq dataset. We thank A. Güler, A. Pathak, S. Kumar, E. Stepanova, S. Chandra and M. Hunter-Chang for feedback and critical evaluation of the data and manuscript. We thank the University of Virginia Flow Cytometry Core, RRID: SCR_017829 for technical assistance. The authors acknowledge Research Computing at the University of Virginia for providing computational resources and technical support that have contributed to the results reported in this publication (https://rc.virginia.edu). We acknowledge the Keck Center for Cellular Imaging for the use of the Zeiss 880/980 multi-photon Airy scan microscopy system (PI- AP; NIH-OD025156).
Author information
Authors and Affiliations
Contributions
A.B.K., A.L.V., I.C.G., C.D.D. and E.R.Z. planned all experiments. A.B.K., I.C.G. and L.J. collected tissue and performed single-cell dissociations. A.L.V. validated all antibodies in the DRG panel. A.B.K. and E.R.Z. performed the mass cytometry measurements. O.Y.C. performed the RNAscope. A.B.K., A.L.V., C.M.W., S.M.G., A.K.H., K.I.F., E.A.P. and E.R.Z. wrote the scripts for the analysis pipeline. A.B.K. and S.A.V. performed data analysis. S.M.G. conceived of and performed the comparisons with scRNA-seq. E.R.Z. and C.D.D. conceived and supervised all aspects of the project. A.B.K., C.D.D. and E.R.Z. prepared figures, and A.B.K., I.C.G., C.D.D. and E.R.Z. wrote the manuscript, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Validation of antibodies for mass cytometry.
All antibodies validated and included in the DRG mass cytometry antibody panel. a) Each antibody was titrated across a range of concentrations (for example 9 μg/ml to 0.01 μg/ml). Known-positive and known-negative control cell samples were tailored for each antibody. Sometimes these were separate samples, and sometimes the known-positive and known-negative cells coexisted in a single sample, distinguishable by a separate antibody counterstain. Optimal staining concentrations for each antibody were determined by identifying the largest difference in signal intensity between known-positive and known-negative cells. b) Biaxials scatterplots for each antibody except for anti-Islet1 (y-axis) in the panel at each age by Islet1 (x-axis), demonstrating positive and negative staining across the DRG developmental time course. Samples were selected from Set 1. For postnatal ages the female sample from Set 1 was used. Full data available in Data Availability.
Extended Data Fig. 2 Pre-processing of DRG samples for mass cytometry.
a) Calibration bead normalization of the raw mass cytometry data (stored as .fcs files) using the Matlab software described in Finck. et al35. b) Clean up gating done with Cytobank (cytobank.org) to remove low quality events from the dataset. Biaxial gates as follows (from left to right, top to bottom): 1) barcode_separation x barcode_negative and 2) barcode_separation x malahoidis_distance removes events that cannot be confidently separated by barcode label; 3) event_length x center and 4) width x center remove events that fall outside of normal Gaussian parameter distribution – these events are often enriched for cell doublets; 5) intercalator x cisplatin removes both non-cell events (for example cellular debris) and dying cells; 6–11) unused metals x intercalator removes high background events. A twelfth clean up gate was required for samples from barcode Set 3 to remove a runtime-dependent increase in background in a subset of channels: time x NeuN. c) Batch correction was run to normalize signal strength between runs (Schuyler et al.)37. Each barcode set included a ‘universal’ sample consisting of excess samples from across the DRG time course. These excess cells were pooled together, and then aliquoted and stored at −80 °C, to be included with each mass cytometry run as an unvarying control. After all samples were run, the universal samples between barcode sets were batch corrected to be as similar as possible on a per-marker basis, and then the batch adjustment process corrected the rest of the samples in that barcode set based on its corresponding universal sample. Arcsinh transformation values were manually adjusted to provide the greatest contrast between background and physiological values. d) A gating hierarchy of major populations found by high dimensional analysis of the whole time course. This gating hierarchy recapitulates the delineation of general cell types identified through high dimensional analysis with UMAP and leiden clustering.
Extended Data Fig. 3 High dimensional analysis of the entire somatosensory time course data set.
a) UMAP embedding of all ‘Cells to Analyze’ (cleanup gating applied) from the whole DRG time course. b) UMAP plot from (a) colored by age. c) UMAP plot grayed out except for low complexity Cluster 6. d) Cells from all ages in Cluster 6, with predominant contribution from E11.5. e) UMAP embedding of Cluster 6 after extraction and secondary Leiden clustering. f) UMAP plot from (e), colored by age. g) Unmodified UMAP embedding from Fig. 1c. UMAP layout was rotated and white space removed for improved visualization. h) UMAP plot from (g) colored by age. i) UMAP plot from (g) colored by expression level for every marker in the DRG antibody panel. j) Violin plots of all markers for all clusters; Fig. 1e is truncated to show just the most salient markers for the general populations. k) Analysis of the variability of each cluster by each sample at each age. The number of cells assigned to each cluster in each sample at each age was determined and the relative abundance of each cluster per sample per age calculated. Line and error bars denote the standard error of the mean. l) The standard error of the mean of the percentage of all cells for each cluster is shown, but only during the ages where the average abundance of that cluster comprised 1% or more of the cells at that age. Note, three clusters (Endothelia 3, Unassigned cells 1, and Unassigned cells 2) never reached that threshold.
Extended Data Fig. 4 Extraction of single age neuron sets for comparison to IHC.
a) Neuronal clusters were extracted from the DRG time course (Fig. 1c), b) and then subjected to secondary Leiden clustering and UMAP embedding. c) Then, individual ages matched to IHC samples were further extracted and subjected to tertiary Leiden clustering and UMAP embedding.
Extended Data Fig. 5 High dimensional analysis of all glial cells and precursors.
a) Cell number per age for all glia and glial precursors. b) Unmodified UMAP embedding from Fig. 3a. White space was trimmed for ease of visualization. c) UMAP plot from (b) colored by age. d) Violin plot of all markers for the glial clusters (from Fig. 3a) e) During pseudotime calculation, several simulations are run, allowing pseudotime to be calculated from these iterations. For ideal pseudotime stability (for example decreased change in cell pseudotime with increasing runs) we assessed the number of runs required to approach an asymptote. We determined 500 simulations was sufficient to reach a stability asymptote. f) We next assessed the distribution of pseudotime by real age (E11.5 to P4). There is a general progression across pseudotime with age with overlap between stages, as expected. g) UMAP plot colored by pseudotime value for all 63,796 downsampled cells included in this analysis. h) UMAP plot from (g) colored by URD segment. i) URD dendrogram of the 4 general populations colored by segment. j) Representative brightfield image of E15.5 sample on a hemocytometer quantified in Fig. 3p. Scale bar, 500 μm.k) Biaxial scatterplots from only putative phagocytic glia showing expression of 4 neuronal markers (TrkA,TrkC, TuJ1, and Islet1), by satellite glial cell marker BFABP at E13.5, E15.5, and P0. l) Biaxial scatterplots comparing marker expression for several neuronally expressed markers (Islet1, NeuN, PGP9.5, TuJ1, TrkA, TrkB, and TrkC) and glial markers (Sox10, TrkB, and Vimentin) by BFABP between only putative phagocytic glia and all Islet1+ cells (neurons and putative phagocytic glia) from all ages.
Extended Data Fig. 6 High dimensional analysis of all neurons.
a) Leiden clustering (LC) and UMAP embedding of neurons extracted from the whole time course, labeled by cell type. Circles indicate the 3 main neuronal subtypes by RTK expression: TrkA+;Ret+, TrkB+, and TrkC+, respectively. b) LC and UMAP of the TrkA+;Ret+ neurons, extracted from a). Three clusters that did not exhibit neuronal markers were removed from the dataset before a final round of LC and UMAP (plot on right). c) LC and UMAP of the TrkB+ neurons extracted from a). d) LC and UMAP of the TrkC+ neurons extracted from a). Putative phagocytic glia expressing TrkC+ could not be removed from TrkC+ neurons in previous analytic iterations, but this could be done at this resolution resulting in a ‘cleaned’ TrkC+ neuronal clustering and UMAP (plot on right). e) TrkA+;Ret+, TrkB+, and TrkC+ UMAP plots colored by age. f) Violin plots of all markers for TrkA+;Ret+, TrkB+, and TrkC+ neurons. g) Key markers in our panel that allow identification of somatosensory DRG populations5. h) UMAP plots colored by expression for all panel markers for TrkA+;Ret+, TrkB+, and TrkC+ neurons. i) TrpM8 transcript data from Usoskin et al.2 showing that TrpM8-expressing neurons are a subset of peptidergic nociceptors. j) UMAP plot of TH+ cells that were extracted from Fig. 4a (Cluster 6) and reclustered. k) UMAP plot of the TH+ cells from P4 overlaid on the grayed out UMAP plot from j). These were the cells used in the comparison to the Usoskin et al2 transcript data in Fig. 4i-n. l) UMAP plot from j) colored by age. m) Violin plot of marker expression of all clusters from j). n) Comparison of TH+ C-LTMR transcripts expression to protein expression for all markers in the mass cytometry panel2. In both cases, transcript or protein expression in C-LTMRs was normalized to all nociceptors.
Extended Data Fig. 7 Analysis of inter-replicate variability for TrkA+, TrkB+, and TrkC+ neuron subclusters.
a) Analysis of the variability of each cluster by each sample for each age for the TrkA+/Ret+ neuron subcluster. The number of cells assigned to each cluster in each sample at each age was determined and the relative abundance of each compared to all neurons in the TrkA+/Ret+ cluster was calculated. b) The standard error of the mean of the percentage of all neurons from the TrkA+/Ret+ dataset for each TrkA+/Ret+ cluster is shown, but only during the ages where the average proportion of that cluster comprised 1% or more of the cells at that age. c) UMAP where TrkA+/Ret+ subclusters have been grouped together by similar expression of markers into a more general cell type. d) UMAP where TrkA+/Ret+ subclusters were recolored by general groups expressing similar markers as denoted in (c). e) The relative abundance for the general groups created in (d) are shown with the samples deemed ‘poorer quality’ (see Supplementary Table 3) indicated as a red circle. f) The relative abundance for the general groups created in (d) shown without the ‘poorer quality’ samples. g) The standard error of the mean of the percentage of all neurons from the grouped TrkA+/Ret+ dataset with the ‘poorer quality’ samples data removed for each TrkA+/Ret+ cluster is shown, but only during the ages where the average proportion of that cluster comprised 1% or more of the cells at that age. h-n) The same analysis as (a-g) except for TrkB+ neurons. (o-u) The same analysis as (a-g) except for TrkC+ neurons. v) Frequency distribution of the S.E.M % of either all cells or neurons for 1) the whole time course (from Extended Data Fig. 3k, l), 2) all neuron subclusters, 3) subclusters grouped by general cell types, and 4) these general cell types with the ‘poorer quality’ samples removed.
Extended Data Fig. 8 URD pseudotime analysis of all neurons.
We initially defined tips as clusters present with ≥ 1% at P4 and all clusters at E11.5 as the root. However, this produced excessively branched pseudotime dendrograms for both a) TrkB+;TrkC+ and b) TrkA+;Ret+ where intermediate and more mature cell states were paired as tips. For instance, TrkA+/Ret+ clusters 5 and 13 are presumptively immature nonpeptidergic nociceptors. The presence of these immature cell types at P4 is expected as cell populations mature over development. By removing presumptive intermediates as tips, we were able to produce the most appropriate molecular trajectory across pseudotime, such as Fig. 5a. c-f) Supplemental URD analysis, same as in Extended Data Fig. 5: c, d) pseudotime stability to calculate simulation number, pseudotime by stage, UMAP of pseudotime value for all 39,944 (TrkB+;TrkC+) not downsampled and 64,997 (TrkA+/Ret+) downsampled cells included in this analysis, UMAP colored by URD segment, for TrkB+;TrkC+ and TrkA+/Ret+ datasets, respectively, and e, f) URD dendrogram colored by URD segment for TrkB+;TrkC+ and TrkA+/Ret+ datasets, respectively. g-t) Heatmaps for all URD tips from TrkB+;TrkC+ and TrkA+/Ret+ datasets, respectively, for all markers.
Extended Data Fig. 9 Multi-Trk+ neurons exhibit altered protein expression.
a) Biaxial scatterplots showing the neurons that express at least two Trks for all 3 combinations: TrkA;TrkB, TrkA;TrkC, and TrkB;TrkC. b) Violin plots of Trk expression for all single-Trk+ neurons (Fig. 4a-c) compared to multi-Trk+ neurons. c) Proportion of TrkA+;TrkB+, TrkA+;TrkC+, and TrkB+;TrkC+ populations at different threshold values: left, TrkA>1.5, TrkB>2.5, TrkC>1.5 and right, TrkA>2, TrkB>3, TrkC>2. d) Proportion of TrkA+;TrkB+, TrkA+;TrkC+, and TrkB+;TrkC+ populations at E11, E12, E15, P0, P5, and P40 from Sharma et al19 scRNA-seq. All cells with transcript expression values >0 were designated as expressing the given transcript. e) Proportion of TrkA+;TrkB+, TrkA+;TrkC+, and TrkB+;TrkC+ populations at P42-56 from Usoskin et al2. Transcript expression was determined by thresholding as in Usoskin et al2: briefly, the three cells with the highest expression for a given transcript were averaged and then multiplied by 5%. Cells with transcript expression greater than this threshold are designated as expressors. f) Proportion of all multi-Trk+ neurons combined at each age across the whole time course from the mass cytometry dataset. g, h) URD dendrograms from Fig. 5a without the URD dataset cells. Instead, each multi-Trk+ neuron type are mapped on the URDs, colored by the age of each cell, over the TrkB;TrkC URD (d) and the TrkA;Ret URD (e). Multi-Trk+ neuron numbers were counted and compared to the number of cells within each TrkB;TrkC URD segment (d). However, the TrkA+ and Ret+ neurons were downsampled (64,997 out of 492,982 neurons). Thus we multiplied the number of neurons in each segment of the TrkA;Ret URD by the downsampling coefficient (7.584689) before determining the proportion of multi-Trk+ neurons over the URD (e). i, j) All p Values for Fig. 6l, m, respectively.
Extended Data Fig. 10 Mass cytometry and scRNA-seq comparison.
a) FLOW-MAP plots from Fig. 7b, c, colored by each protein marker in the DRG antibody panel for comparison between the mass cytometry and the scRNA-seq19. In cases where expression was not detected in the scRNA-seq dataset, the FLOW-MAPs are colored a single intermediate color (for example as in GAD2, GFAP, etc). Markers are named with the gene name. b) Graphed expression levels for all markers in the protein panel for TrkA+, TrkB+, and TrkC+ populations compared between mass cytometry and scRNA-seq.
Supplementary information
Supplementary Table 1
Antibody panel tailored for DRG development. Each antibody or reagent used in the mass cytometry staining panel is listed with antigen, conjugated metal, manufacturer, product number, antibody clone and concentration. Antibodies recognizing surface proteins were stained before methanol permeabilization in a single master mix, and intracellular proteins, including nuclear proteins, were stained in a single master mix after permeabilization, denoted by surface or intracellular stain protocol, respectively.
Supplementary Table 2
Sample data. Every sample included in this study is reported. For each sample, this includes the age of the mice, the date the cells were collected, the genotype, the number of animals in the sample, the sex for postnatal samples (all prenatal litters were not sex separated), the total cell count and which mass cytometry run ‘set’ the sample belonged to. Color indicates barcode set (blue = barcode set 1, green = barcode set 2, red = barcode set 3).
Supplementary Table 3
Efficiency across data clean-up. Data event counts for each sample across the three mass cytometry barcode sets for the full time course tracked from mass cytometry reads through pre-processing gating clean-up. Data events were first analyzed for the first two barcode sets, each comprising one replicate sample set of a complete time course from E11.5 to P4. These samples are first gated by barcode stringency to ensure that samples without a clear and clean barcode label are removed. Then, data events outside of normal parameters for a mass cytometry read are gated out. The remaining events are gated by the viability dye cisplatin and DNA intercalator Iridium to remove damaged cells or non-single cells. Finally, empty isotope channels are gated to remove events with excessive background to produce a population of cleaned data titled ‘Cells to Analyze’. An Islet1+ gate enabled an estimate on the percentage of neurons at each age. Using these initial numbers, we determined a handful of ages to collect additional samples (barcode set 3). Barcode set 3 was also analyzed across gating clean-up.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keeler, A.B., Van Deusen, A.L., Gadani, I.C. et al. A developmental atlas of somatosensory diversification and maturation in the dorsal root ganglia by single-cell mass cytometry. Nat Neurosci 25, 1543–1558 (2022). https://doi.org/10.1038/s41593-022-01181-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01181-8