Abstract
Obesity is a global pandemic that is causally linked to many life-threatening diseases. Apart from some rare genetic conditions, the biological drivers of overeating and reduced activity are unclear. Here, we show that neurotensin-expressing neurons in the mouse interstitial nucleus of the posterior limb of the anterior commissure (IPAC), a nucleus of the central extended amygdala, encode dietary preference for unhealthy energy-dense foods. Optogenetic activation of IPACNts neurons promotes obesogenic behaviors, such as hedonic eating, and modulates food preference. Conversely, acute inhibition of IPACNts neurons reduces feeding and decreases hedonic eating. Chronic inactivation of IPACNts neurons recapitulates these effects, reduces preference for sweet, non-caloric tastants and, furthermore, enhances locomotion and energy expenditure; as a result, mice display long-term weight loss and improved metabolic health and are protected from obesity. Thus, the activity of a single neuronal population bidirectionally regulates energy homeostasis. Our findings could lead to new therapeutic strategies to prevent and treat obesity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are contained in the main text, Extended Data or Supplementary Information. Source data are provided with this paper.
Code availability
Custom code is available on GitHub at https://github.com/Alefurlan/IPACpaper.
References
Bluher, M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298 (2019).
Fenselau, H. et al. A rapidly acting glutamatergic ARC→PVH satiety circuit postsynaptically regulated by α-MSH. Nat. Neurosci. 20, 42–51 (2017).
Li, M. M. et al. The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653–667 (2019).
Zhang, X. & van den Pol, A. N. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356, 853–859 (2017).
Speakman, J. R. et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis. Model Mech. 4, 733–745 (2011).
Trexler, E. T., Smith-Ryan, A. E. & Norton, L. E. Metabolic adaptation to weight loss: implications for the athlete. J. Int Soc. Sports Nutr. 11, 7 (2014).
Hill, J. O., Wyatt, H. R. & Peters, J. C. Energy balance and obesity. Circulation 126, 126–132 (2012).
Rossi, M. A. & Stuber, G. D. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 27, 42–56 (2018).
Alheid, G. F. Extended amygdala and basal forebrain. Ann. N. Y. Acad. Sci. 985, 185–205 (2003).
Tanaka, D. H., Li, S., Mukae, S. & Tanabe, T. Genetic access to gustatory disgust-associated neurons in the interstitial nucleus of the posterior limb of the anterior commissure in male mice. Neuroscience 413, 45–63 (2019).
Tanaka, D. H., Li, S., Mukae, S. & Tanabe, T. Genetic recombination in disgust-associated bitter taste-responsive neurons of the central nucleus of amygdala in male mice. Neurosci. Lett. 742, 135456 (2021).
Gehrlach, D. A. et al. A whole-brain connectivity map of mouse insular cortex. eLife 9, e55585 (2020).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Shammah-Lagnado, S. J., Alheid, G. F. & Heimer, L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J. Comp. Neurol. 439, 104–126 (2001).
Steculorum, S. M. et al. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165, 125–138 (2016).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
O’Connor, E. C. et al. Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 (2015).
Tan, H. E. et al. The gut–brain axis mediates sugar preference. Nature 580, 511–516 (2020).
Yeomans, M. R. Taste, palatability and the control of appetite. Proc. Nutr. Soc. 57, 609–615 (1998).
Patel, J. M. et al. Sensory perception drives food avoidance through excitatory basal forebrain circuits. eLife 8, e44548 (2019).
Riera, C. E. et al. The sense of smell impacts metabolic health and obesity. Cell Metab. 26, 198–211 (2017).
Cabanac, M. Physiological role of pleasure. Science 173, 1103–1107 (1971).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Murray, A. J. et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat. Neurosci. 14, 297–299 (2011).
Strekalova, T., Spanagel, R., Dolgov, O. & Bartsch, D. Stress-induced hyperlocomotion as a confounding factor in anxiety and depression models in mice. Behav. Pharmacol. 16, 171–180 (2005).
Trajcevski, K. E. et al. Enhanced lipid oxidation and maintenance of muscle insulin sensitivity despite glucose intolerance in a diet-induced obesity mouse model. PLoS ONE 8, e71747 (2013).
O’Neal, T. J., Friend, D. M., Guo, J., Hall, K. D. & Kravitz, A. V. Increases in physical activity result in diminishing increments in daily energy expenditure in mice. Curr. Biol. 27, 423–430 (2017).
Berthoud, H. R. & Munzberg, H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol. Behav. 104, 29–39 (2011).
Chen, Y., Lin, Y. C., Kuo, T. W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Lowell, B. B. New neuroscience of homeostasis and drives for food, water, and salt. N. Engl. J. Med. 380, 459–471 (2019).
Terral, G. et al. CB1 receptors in the anterior piriform cortex control odor preference memory. Curr. Biol. 29, 2455–2464 (2019).
Xu, W. & Wilson, D. A. Odor-evoked activity in the mouse lateral entorhinal cortex. Neuroscience 223, 12–20 (2012).
Bitzenhofer, S. H., Westeinde, E. A., Zhang, H. B. & Isaacson, J. S. Rapid odor processing by layer 2 subcircuits in lateral entorhinal cortex. eLife 11, e75065 (2022).
de Araujo, I. E. et al. Food reward in the absence of taste receptor signaling. Neuron 57, 930–941 (2008).
Beeler, J. A. et al. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur. J. Neurosci. 36, 2533–2546 (2012).
Urban, D. J. et al. Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41, 1404–1415 (2016).
Blaha, C. D. & Phillips, A. G. Pharmacological evidence for common mechanisms underlying the effects of neurotensin and neuroleptics on in vivo dopamine efflux in the rat nucleus accumbens. Neuroscience 49, 867–877 (1992).
Woodworth, H. L. et al. Neurotensin receptor-1 identifies a subset of ventral tegmental dopamine neurons that coordinates energy balance. Cell Rep. 20, 1881–1892 (2017).
Woodworth, H. L., Brown, J. A., Batchelor, H. M., Bugescu, R. & Leinninger, G. M. Determination of neurotensin projections to the ventral tegmental area in mice. Neuropeptides 68, 57–74 (2018).
Aldiss, P. et al. Exercise-induced ‘browning’ of adipose tissues. Metabolism 81, 63–70 (2018).
Shimizu, I. et al. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Invest. 124, 2099–2112 (2014).
Recena Aydos, L. et al. Nonalcoholic fatty liver disease induced by high-fat diet in C57BL/6 models. Nutrients 11, 3067 (2019).
Stephenson-Jones, M. et al. A basal ganglia circuit for evaluating action outcomes. Nature 539, 289–293 (2016).
Zhang, X. & Li, B. Population coding of valence in the basolateral amygdala. Nat. Commun. 9, 5195 (2018).
Gamba, O. F. M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330 (2016).
Xiao, X. et al. A genetically defined compartmentalized striatal direct pathway for negative reinforcement. Cell 183, 211–227 (2020).
Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U. & Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8, 1149–1154 (2013).
Stephenson-Jones, M. et al. Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron 105, 921–933 (2020).
Acknowledgements
We thank T. Russo for technical assistance and members of the Li laboratory for helpful discussions. This work was supported by grants from EMBO (ALTF 458–2017, A.F.), the Swedish Research Council (2017-00333, A.F.), the Charles H. Revson Senior Fellowship in Biomedical Science (19–23, A.F.), the National Institutes of Health (R01MH101214, R01MH108924, R01DA050374 and R01NS104944, B.L.), the Cold Spring Harbor Laboratory and Northwell Health Affiliation (B.L.), the Feil Family Neuroscience Endowment (B.L.) and the German Academic Scholarship Foundation (E.C.G.).
Author information
Authors and Affiliations
Contributions
A.F. and B.L. conceived and designed the study. A.F. conducted the experiments and analyzed data. A.C. assisted with the photometry experiments with food odors and the data analysis. S. Boyle set up behavioral rigs and generated MATLAB code for controlling behavioral devices and analyzing photometry data. R.S. assisted with the smFISH experiments. R.R. and J.H. assisted with operating the metabolic cages. E.C.G. assisted with the GTT and ITT experiments. R.S. and E.C.G. collected tissue samples and performed quantitative PCR experiments. J.G. assisted with the EPM and OF experiments. S. Beyaz provided critical reagents. T.J. supervised the experiments by E.C.G. and assisted with interpreting metabolic data. S.D.S. supervised the experiments by A.C. and assisted with analyzing and interpreting the data. A.F. and B.L. wrote the paper with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Roger Adan, Alexander Nectow, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 IPACNts neurons encode the hedonic value of a stimulus.
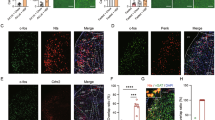
(a) Schematics showing the locations of optic fiber placement in the mice used in Figs. 2 and 3. (b) Feeding behavior of mice when presented with HFD (green) or chow (orange), for 20 minutes, in food restricted (FR, left) or sated condition (right). N = 8. Group effect: F(1,7)=95.19, p < 0.0001, **p < 0.01; ****p < 0.0001; Two-way RM ANOVA, Sidak’s test. (c, d) Schematics of the experimental setup (c) and task structure (d) used in Fig. 2. Bottom panel: representative raster plot showing licking behavior following liquid delivery. (e) Drinking behavior of wild-type mice when presented with Intralipid 0.5% (Fat 0.5%, green) or water (orange), in a 2-bottle preference test, for 72-h, in sated condition. N = 6 mice. ***P = 0.0003, paired t-test. (f-h) Food-restricted (FR) mice (f) and water-restricted (WR) mice (g, h) were presented with equal volumes of liquids in the same session. Left: Area under the curve (AUC) of GCAMP6f signals. Right: licking behavior (behavior) of mice. AUC and licking behavior were measured in a 3-s window following the first lick. Paired t-tests, n = 5 mice/group in all panels. (f) Sucrose (green) or sucralose (orange); AUC: p = 0.1071 (n.s.); Behavior: *p = 0.0462. (g) Monosodium glutamate (MSG, green) or water (orange); AUC: p = 0.3008 (n.s.); Behavior: p = 0.7061 (n.s.). (h) Citric acid (green) or water (orange); AUC: p = 0.1997 (n.s.); Behavior: p = 0.8677 (n.s.).
Extended Data Fig. 2 Response of IPACNts neurons to odors from several diets.
(a, b) Heatmaps of the response of IPACNts neurons in individual mice to odors derived from different food sources, under sated or food-restricted condition, as indicated. Dashed lines indicate the onset of odor presentation. (c) Average GCaMP6f signals from IPACNts neurons in food-restricted mice aligned to the presentation of different odors (dashed line). (d) Area under the curve (AUC) of the responses in individual mice in (c) in a 3-s window following odor presentation. N = 4 mice. F(3,9)=10.36, p = 0.0028; *p < 0.05, **p < 0.01, n.s., p > 0.05; one-way RM ANOVA, Holm-Sidak’s test. (e) Average GCaMP6f signals from IPACNts neurons in sated mice aligned to odor presentation (dashed line). (f) Area under the curve (AUC) of the responses in individual mice in (e) in a 3-s window following odor presentation. N = 5 mice. F(3,12)=5.169, p = 0.0160; *p < 0.05, p > 0.05 (n.s); one-way RM ANOVA, Holm-Sidak’s test.
Extended Data Fig. 3 Characterization of behavioral effects following activation of IPACNts neurons.
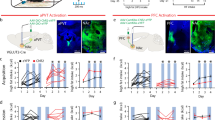
(a) Optic fiber placement for mice in Fig. 4. (b) Effect of photostimulation of IPACNts neurons in mice fed dark chocolate (DCh), chow, sucrose, HFD, white chocolate (WCh). ChR2 (n = 9) or GFP (n = 6) for DCh, ChR2 (n = 9) or GFP (n = 8) for chow, sucrose, HFD, WCh. Two-way RM ANOVA, Sidak’s test. DCh, group effect: F(1,13)=7.374, p = 0.0177; chow, group effect: F(1,15)=8.999, p = 0.0090; sucrose, group effect: F(1,15)=7.829, p = 0.0135; HFD, group effect: F(1,15)=21.22, p = 0.0003; WCh, group effect: F(1,15)=22.56, p = 0.0003. (c) Effect of photostimulation of IPACNts neurons in mice presented with an inedible pencil eraser. ChR2 (n = 9) or GFP (n = 6). two-way RM ANOVA, Sidak’s test. Gnawing, interaction effect: F(2,26)=4.939, p = 0.0152; intake: F(2,26)=1.066, p = 3591 (n.s.). ****p < 0.0001; p > 0.05(n.s.). (d, e) Photostimulation of IPACNts neurons increased the number (d) and the duration (e) of feeding bouts in ChR2 (n = 9) but not GFP mice (n = 7). (d) GFP: *p = 0.0465, ChR2: **p = 0.0028; (e) GFP: p = 0.0982(n.s.), ChR2: ***p = 0.0007, paired t-test. (f) Effect of photostimulation of IPACNts neurons in ChR2 mice fed quinine-flavored or plain chow pellets (n = 5). Interaction effect: F(2,8)=9.476, p = 0.0078, **p < 0.01, two-way RM ANOVA, Sidak’s comparisons test. (g) Effect of photostimulation of IPACNts neurons on liquid consumption (control for Fig. 4e). GFP mice (n = 5), interaction effect: F (4, 16)=1.119, p = 0.3820 (n.s.). Two-way RM ANOVA. (h) Self-stimulation paradigm (left) and quantification of the poking responses of ChR2 (n = 9) and GFP mice (n = 8). Group effect: F(1,15)=37.63, p < 0.0001; ****p < 0.0001; p > 0.05 (n.s.), two-way RM ANOVA, Sidak’s test. (i) Distance traveled in the RTPP/A task. ChR2 (n = 11) and GFP (n = 8) mice. Group effect: F(1,17)=34.11, p < 0.0001; ****p < 0.0001; p > 0.05 (n.s.), two-way RM ANOVA, Sidak’s test. (j) Distance traveled in the open field test. ChR2 (n = 8) and GFP (n = 6) mice. Group effect: F(1,12)=17.30, p = 0.0013; ****p < 0.0001; p > 0.05 (n.s.) two-way RM ANOVA, Sidak’s test.
Extended Data Fig. 4 Inactivation of IPACNts neurons impairs hedonic perception.
(a) HFD intake over a 2-h period in sated NtsCre mice expressing mCherry (gray, control) or KORD (red) injected with DMSO or SalB. mCherry mice, n = 7; KORD mice, n = 5. paired t-test. Cherry DMSO-SaLB: p = 0.7748, (n.s.); KORD DMSO-SaLB: p = 0.1066 (n.s.). Paired t-test.(b) Percentage change of HFD intake in food-restricted mice expressing mCherry (gray, control) or KORD (red) when injected with SaLB, normalized to their intake when injected with DMSO, within 30 minutes from food presentation. mCherry mice, n = 7; KORD mice, n = 5. *p = 0.0326. Unpaired t-test. (c) Daily water intake of the GFP mice (n = 10) and TeLC mice (n = 8) fed chow. p = 0.8023 (n.s.), unpaired t-test. (d) Daily water intake of the GFP mice (n = 10) and TeLC mice (n = 8) fed HFD. *p = 0.0305, unpaired t-test. (e) Schematic of the 2-bottle preference test (left) for sucralose (center) and sucrose (right). Sucralose: GFP mice (n = 5), TeLC mice (n = 5): **p = 0.0055, unpaired t-test. Sucrose: GFP mice (n = 6); TeLC mice (n = 5); p = 0.6488 (n.s.), unpaired t-test. Legend: L, left bottle, R, right bottle. (f) Comparison of energy intake from chow and HFD diets (derived from Fig. 5i and j). GFP (n = 10): ****p < 0.0001; TeLC mice (n = 8): p = 0.3562 (n.s.); paired t-test. (g) Change in energy intake after the switch from chow to HFD. ***P = 0.0002, unpaired t-test.
Extended Data Fig. 5 Inactivation of IPACNts neurons has positive metabolic effects.
(a) Changes in body weight (BW) following injection (d0). GFP mice (n = 11): F(3, 30)=6.588, p = 0.0015; **p < 0.01; ***p < 0.001; TeLC mice (n = 10): F(3, 27)=28.11, p < 0.0001; ****p < 0.0001, p > 0.05 (n.s.); one-way RM ANOVA, Sidak’s test. (b) Volume of carbon dioxide produced (VCO2) by GFP (n = 10) and TeLC mice (n = 8). Group effect: F(1, 16)=5.745, p = 0.0291, two-way RM ANOVA. (c) Average carbon dioxide production (VCO2) of the mice in (b). GFP (n = 10); TeLC (n = 8). Group effect: F(1, 16)=5.603, p = 0.0309; *p < 0.05, p > 0.05 (n.s.); two-way RM ANOVA, Sidak’s test. (d) Respiratory exchange ratio (RER) of GFP (n = 10) and TeLC mice (n = 8). Interaction effect: F(70,1120) = 5.042, p < 0.0001, two-way RM ANOVA. (e) Average RER of GFP (n = 10) and TeLC mice (n = 8) fed chow. Interaction effect: F(1, 16)=7.546, *p = 0.0143; two-way RM ANOVA, Sidak’s test. (f) Average energy expenditure of GFP (n = 10) and TeLC mice (n = 8) fed HFD. Group effect: F(1, 16)=6.526, p = 0.0212; *p < 0.05, n.s., p > 0.05; two-way RM ANOVA, Sidak’s test. (g) Average oxygen consumption (VO2) of GFP (n = 10) and TeLC mice (n = 8) fed HFD. Group effect: F(1, 16)=6.066, *p = 0.0255, p > 0.05 (n.s.); two-way RM ANOVA, Sidak’s test. (h) Average carbon dioxide production (VCO2) of GFP (n = 10) and TeLC mice (n = 8) fed HFD. Group effect: F(1, 16)=5.276, *p = 0.0355, p > 0.05 (n.s.); two-way RM ANOVA, Sidak’s test. (i) Average locomotor activity of GFP (n = 10) and TeLC mice (n = 8) fed HFD. Group effect: F(1,16)=25.21, p < 0.0001; ****p < 0.0001, p > 0.05 (n.s.), two-way RM ANOVA, Sidak’s test.
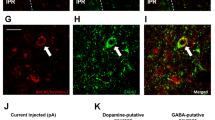
Extended Data Fig. 6 Network of IPACNts neurons.
(a) Representative images of brain areas innervated by IPACNts (green) and mBSTNts (red) neurons. Scale bar: 100 μm. STLV: ventral lateral division of the BNST; STMA: anterior medial division; VP: ventral pallidum; CPu: caudate putamen; IPAC: interstitial nucleus of the posterior limb of the anterior commissure (aca); LC: locus coeruleus; PBN: parabrachial nu.; CeA: central amygdala; BLA: basolateral amygdala; PAG: periacqueductal gray; DR: dorsal raphe. (b) Representative image of smFISH for Nts on retrograde labelled CT-B+ neurons in the IPAC. The square in the image show the high-magnification area showed in Fig. 7h (right). Scale bar: 100 μm. (c) Schematics showing the locations of optic fiber placement in the mice used in Fig. 7. (d) Effect of light delivery into the IPAC of the ChR2 (n = 9) or GFP (n = 5) mice on gnawing (left) and consumption (right) of inedible items (that is, pencil eraser). Gnawing (group effect): F(1,12)=11.51, p = 0.0053, two-way RM ANOVA, Sidak’s test. ****p < 0.0001. Intake: (group effect): F(1,12)=0.5327, p = 4783, two-way RM ANOVA. (e) Preference of ChR2 (n = 8) and GFP mice (n = 6) for the left chamber side. Interaction effect: F(2,24)=125.1; p < 0.0001; ****p < 0.0001; p > 0.05 (n.s.). Two-way RM ANOVA, Sidak’s test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Furlan, A., Corona, A., Boyle, S. et al. Neurotensin neurons in the extended amygdala control dietary choice and energy homeostasis. Nat Neurosci 25, 1470–1480 (2022). https://doi.org/10.1038/s41593-022-01178-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01178-3
This article is cited by
-
Current perspectives on brain circuits involved in food addiction-like behaviors
Journal of Neural Transmission (2024)