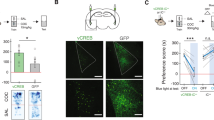
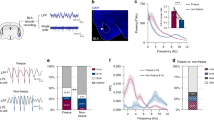
Abstract
Behavioral flexibility—that is, the ability to deviate from established behavioral sequences—is critical for navigating dynamic environments and requires the durable encoding and retrieval of new memories to guide future choice. The orbitofrontal cortex (OFC) supports outcome-guided behaviors. However, the coordinated neural circuitry and cellular mechanisms by which OFC connections sustain flexible learning and memory remain elusive. Here we demonstrate in mice that basolateral amygdala (BLA)→OFC projections bidirectionally control memory formation when familiar behaviors are unexpectedly not rewarded, whereas OFC→dorsomedial striatum (DMS) projections facilitate memory retrieval. OFC neuronal ensembles store a memory trace for newly learned information, which appears to be facilitated by circuit-specific dendritic spine plasticity and neurotrophin signaling within defined BLA–OFC–DMS connections and obstructed by cocaine. Thus, we describe the directional transmission of information within an integrated amygdalo-fronto-striatal circuit across time, whereby novel memories are encoded by BLA→OFC inputs, represented within OFC ensembles and retrieved via OFC→DMS outputs during future choice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Individual data points are represented throughout. More detailed datasets are available from the corresponding author upon reasonable request.
References
Everitt, B. J. & Robbins, T. W. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 67, 23–50 (2016).
Izquierdo, A. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J. Neurosci. 37, 10529–10540 (2017).
Stalnaker, T. A., Cooch, N. K. & Schoenbaum, G. What the orbitofrontal cortex does not do. Nat. Neurosci. 18, 620–627 (2015).
Gardner, M. P. H., Conroy, J. C., Sanchez, D. C., Zhou, J. & Schoenbaum, G. Real-time value integration during economic choice is regulated by orbitofrontal cortex. Curr. Biol. 29, 4315–4322 (2019).
Parkes, S. L. et al. Insular and ventrolateral orbitofrontal cortices differentially contribute to goal-directed behavior in rodents. Cereb. Cortex 28, 2313–2325 (2018).
Banerjee, A. et al. Value-guided remapping of sensory cortex by lateral orbitofrontal cortex. Nature 585, 245–250 (2020).
Schuck, N. W., Cai, M. B., Wilson, R. C. & Niv, Y. Human orbitofrontal cortex represents a cognitive map of state space. Neuron 91, 1402–1412 (2016).
Wilson, R. C., Takahashi, Y. K., Schoenbaum, G. & Niv, Y. Orbitofrontal cortex as a cognitive map of task space. Neuron 81, 267–279 (2014).
Niv, Y. Learning task-state representations. Nat. Neurosci. 22, 1544–1553 (2019).
Behrens, T. E. J. et al. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018).
Hirokawa, J., Vaughan, A., Masset, P., Ott, T. & Kepecs, A. Frontal cortex neuron types categorically encode single decision variables. Nature 576, 446–451 (2019).
Zhou, J. et al. Rat orbitofrontal ensemble activity contains multiplexed but dissociable representations of value and task structure in an odor sequence task. Curr. Biol. 29, 897–907 (2019).
Groman, S. M. et al. Orbitofrontal circuits control multiple reinforcement-learning processes. Neuron 103, 734–746 (2019).
Namboodiri, V. M. K. et al. Single-cell activity tracking reveals that orbitofrontal neurons acquire and maintain a long-term memory to guide behavioral adaptation. Nat. Neurosci. 22, 1110–1121 (2019).
Malvaez, M., Shieh, C., Murphy, M. D., Greenfield, V. Y. & Wassum, K. M. Distinct cortical–amygdala projections drive reward value encoding and retrieval. Nat. Neurosci. 22, 762–769 (2019).
Barreiros, I. V., Panayi, M. C. & Walton, M. E. Organization of afferents along the anterior–posterior and medial–lateral axes of the rat orbitofrontal cortex. Neuroscience 460, 53–68 (2021).
Schoenbaum, G., Setlow, B., Saddoris, M. P. & Gallagher, M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron 39, 855–867 (2003).
Rudebeck, P. H., Mitz, A. R., Chacko, R. V. & Murray, E. A. Effects of amygdala lesions on reward–value coding in orbital and medial prefrontal cortex. Neuron 80, 1519–1531 (2013).
de Wit, S., Ostlund, S. B., Balleine, B. W. & Dickinson, A. Resolution of conflict between goal-directed actions: outcome encoding and neural control processes. J. Exp. Psychol. Anim. Behav. Process 35, 382–393 (2009).
Dickinson, A., Nicholas, D. J. & Adams, A. D. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Q. J. Exp. Psychol. B 35, 35–51 (1983).
Lucantonio, F., Stalnaker, T. A., Shaham, Y., Niv, Y. & Schoenbaum, G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat. Neurosci. 15, 358–366 (2012).
Ersche, K. D. et al. Carrots and sticks fail to change behavior in cocaine addiction. Science 352, 1468–1471 (2016).
DePoy, L. M., Zimmermann, K. S., Marvar, P. J. & Gourley, S. L. Induction and blockade of adolescent cocaine-induced habits. Biol. Psychiatry 81, 595–605 (2017).
Northoff, G. & Tumati, S. ‘Average is good, extremes are bad’—non-linear inverted U-shaped relationship between neural mechanisms and functionality of mental features. Neurosci. Biobehav. Rev. 104, 11–25 (2019).
Pan, W. X., Mao, T. & Dudman, J. T. Inputs to the dorsal striatum of the mouse reflect the parallel circuit architecture of the forebrain. Front. Neuroanat. 4, 147 (2010).
Gremel, C. M. & Costa, R. M. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat. Commun. 4, 2264 (2013).
Zimmermann, K. S., Yamin, J. A., Rainnie, D. G., Ressler, K. J. & Gourley, S. L. Connections of the mouse orbitofrontal cortex and regulation of goal-directed action selection by brain-derived neurotrophic factor. Biol. Psychiatry 81, 366–377 (2017).
Saddoris, M. P., Gallagher, M. & Schoenbaum, G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron 46, 321–331 (2005).
Lucantonio, F. et al. Neural estimates of imagined outcomes in basolateral amygdala depend on orbitofrontal cortex. J. Neurosci. 35, 16521–16530 (2015).
Berry, K. P. & Nedivi, E. Spine dynamics: are they all the same? Neuron 96, 43–55 (2017).
DeNardo, L. A., Berns, D. S., DeLoach, K. & Luo, L. Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat. Neurosci. 18, 1687–1697 (2015).
Yang, G., Pan, F. & Gan, W. B. Stably maintained dendritic spines are associated with lifelong memories. Nature 462, 920–924 (2009).
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766 (2004).
Chao, M. V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 (2003).
Gallagher, M., McMahan, R. W. & Schoenbaum, G. Orbitofrontal cortex and representation of incentive value in associative learning. J. Neurosci. 19, 6610–6614 (1999).
Pickens, C. L. et al. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J. Neurosci. 23, 11078–11084 (2003).
Noonan, M. P., Chau, B. K. H., Rushworth, M. F. S. & Fellows, L. K. Contrasting effects of medial and lateral orbitofrontal cortex lesions on credit assignment and decision-making in humans. J. Neurosci. 37, 7023–7035 (2017).
Jocham, G. et al. Reward-guided learning with and without causal attribution. Neuron 90, 177–190 (2016).
Panayi, M. C. & Killcross, S. Functional heterogeneity within the rodent lateral orbitofrontal cortex dissociates outcome devaluation and reversal learning deficits. eLife 7, e37357 (2018).
Ostlund, S. B. & Balleine, B. W. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J. Neurosci. 27, 4819–4825 (2007).
Ramirez-Lugo, L., Penas-Rincon, A., Angeles-Duran, S. & Sotres-Bayon, F. Choice behavior guided by learned, but not innate, taste aversion recruits the orbitofrontal cortex. J. Neurosci. 36, 10574–10583 (2016).
Farovik, A. et al. Orbitofrontal cortex encodes memories within value-based schemas and represents contexts that guide memory retrieval. J. Neurosci. 35, 8333–8344 (2015).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22, 460–469 (2019).
Josselyn, S. A. & Tonegawa, S. Memory engrams: recalling the past and imagining the future. Science 367, eaaw4325 (2020).
Wikenheiser, A. M., Marrero-Garcia, Y. & Schoenbaum, G. Suppression of ventral hippocampal output impairs integrated orbitofrontal encoding of task structure. Neuron 95, 1197–1207 (2017).
Zhou, J. et al. Complementary task structure representations in hippocampus and orbitofrontal cortex during an odor sequence task. Curr. Biol. 29, 3402–3409 (2019).
McGaugh, J. L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 27, 1–28 (2004).
Leong, Y. C., Radulescu, A., Daniel, R., DeWoskin, V. & Niv, Y. Dynamic interaction between reinforcement learning and attention in multidimensional environments. Neuron 93, 451–463 (2017).
Niv, Y. et al. Reinforcement learning in multidimensional environments relies on attention mechanisms. J. Neurosci. 35, 8145–8157 (2015).
DePoy, L. M. & Gourley, S. L. Synaptic cytoskeletal plasticity in the prefrontal cortex following psychostimulant exposure. Traffic 16, 919–940 (2015).
Wright, W. J. et al. Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat. Neurosci. 23, 32–46 (2020).
Kanta, V., Pare, D. & Headley, D. B. Closed-loop control of gamma oscillations in the amygdala demonstrates their role in spatial memory consolidation. Nat. Commun. 10, 3970 (2019).
Huff, M. L., Miller, R. L., Deisseroth, K., Moorman, D. E. & LaLumiere, R. T. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl Acad. Sci. USA 110, 3597–3602 (2013).
Jones, J. L. et al. Orbitofrontal cortex supports behavior and learning using inferred but not cached values. Science 338, 953–956 (2012).
Vertechi, P. et al. Inference-based decisions in a hidden state foraging task: differential contributions of prefrontal cortical areas. Neuron 106, 166–176 (2020).
Stalnaker, T. A. et al. Orbitofrontal neurons infer the value and identity of predicted outcomes. Nat. Commun. 5, 3926 (2014).
Takahashi, Y. K. et al. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nat. Neurosci. 14, 1590–1597 (2011).
Hayashi-Takagi, A. et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338 (2015).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15, 1748–1757 (2001).
Dickinson, A. & Balleine, B. Motivational control of goal-directed action. Anim. Learn. Behav. 22, 1–18 (1994).
Vaghi, M. M. et al. Action-outcome knowledge dissociates from behavior in obsessive-compulsive disorder following contingency degradation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 200–209 (2019).
Rossi, M. A. & Yin, H. H. Methods for studying habitual behavior in mice. Curr. Protoc. Neurosci. 60, 8.29.1–8.29.9 (2012).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Gomez, J. L. et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017).
Huang, L. et al. Organizational principles of amygdalar input-output neuronal circuits. Mol. Psychiatry 26, 7118–7129 (2021).
Wang, J. et al. Divergent projection patterns revealed by reconstruction of individual neurons in orbitofrontal cortex. Neurosci. Bull. 37, 461–477 (2021).
Shinonaga, Y., Takada, M. & Mizuno, N. Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience 58, 389–397 (1994).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates 2nd edn (Academic Press, 2001).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Zingg, B. et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017).
Radley, J. J., Anderson, R. M., Hamilton, B. A., Alcock, J. A. & Romig-Martin, S. A. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci. 33, 14379–14391 (2013).
Acknowledgements
We thank R. A. Davies for technical assistance and laboratory members for feedback on the manuscript. This work was supported by National Institutes of Health grants F30MH117873 (D.C.L.), R01MH117103 (S.L.G.) and R01DA044297 (S.L.G.). The Emory Viral Vector Core is supported by National Institute of Neurological Disorders and Stroke Core Facilities grant P30NS055077. The Emory National Primate Research Center is supported by Office of Research Infrastructure Programs grant P51OD011132. Research reported in this publication was also supported, in part, by the Emory University Integrated Cellular Imaging Core and Children’s Healthcare of Atlanta.
Author information
Authors and Affiliations
Contributions
Conceptualization: D.C.L. and S.L.G. Methodology: D.C.L. and S.L.G. Investigation (surgical preparation, behavioral testing and microscopy experiments): D.C.L., N.M.D., B.R.B., E.G.P., B.K. and S.A.B. Formal analysis (including statistical analyses): D.C.L., J.F. and T.L. Writing: D.C.L. and S.L.G. Supervision: S.L.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Laura Bradfield, Stan Floresco and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Mice do not display preference for one nose-poke aperture during training.
Response side bias (responses on aperture to be non-reinforced / total responses) during training sessions. (a) BLA→OFC inactivation: memory encoding (session: F14,378 = 1.22, 0.256; session × CNO: F28,378 < 1), (b) delayed memory encoding (session: F10,80 = 3.18, p = 0.002; session × CNO: F10,80 < 1), or (c) memory retrieval (session: F8,128 = 4.86, p < 0.001; session × CNO: F8,128 < 1). (d) BLA→OFC stimulation (session: F14,392 < 1; session × cocaine: F14,392 < 1; session × CNO: F14,392 = 1.30, p = 0.205; session × cocaine × CNO: F14,392 < 1). (e) OFC→DMS inactivation (session: F8,144 = 1.05, p = 0.404; session× CNO: F8,144 = 1.21, p = 0.295). (f) OFC→BLA inactivation (session: F8,112 = 1.04, p = 0.205; session × CNO: F8,112 < 1). (g) OFC memory trace inactivation: novel (session: F6,96 = 1.03, p = 0.414; session × 4OHT: F6,96 = 1.73, p = 0.122) or (h) familiar reinforcement conditions (session: F6,108 < 1; session × 4OHT: F6,108 < 1). (i) BDNF-dependent circuit function: BLA-OFC (session: F6,204 = 1.10 p = 0.365; session × lateralization: F12,204 < 1) or (j) OFC-DMS disconnections (session: F6,138 = 1.44, p = 0.204; session × lateralization: F12,138 < 1). Data presented as individual points (semi-transparent) and group means (solid). Correspondence to main figures noted.
Extended Data Fig. 2 Nose-poking and lever-pressing actions are instrumental in nature.
(a,e) Behavioral procedure used to assess sensitivity to instrumental omission for nose-poking or lever-pressing. (b, c) Nose-poke responses across training (F6,42 = 10.1, p < 0.001) and during omission session (F5,35 = 16.9, p < 0.001). (d) Raster plot of nose-poking responses for each animal throughout the omission session. (f, g) Lever-pressing across training (F3,21 = 29.1, p < 0.001) and during omission session (F5,35 = 35.0, p < 0.001). (h) Raster plot of lever-pressing responses for each animal throughout the omission session. Data presented as individual points (semi-transparent) and group means (solid). Repeating measures ANOVA was applied, 2-sided, with no adjustment for multiple comparisons required.
Extended Data Fig. 3 Inactivation of posterolateral OFC does not disrupt flexible memory encoding.
(a) Left. Chemogenetic receptor expression in the anterior ventrolateral OFC from experiments described in Figs.1–3 of main text. Right. Extent of inhibitory chemogenetic receptor expression in the posterolateral OFC. Anterior-posterior (A-P) distance from bregma noted. (b) Timing of CNO administration for posterolateral OFC inactivation during memory encoding. (c) Responses across training (session: F6,84 = 73.9, p < 0.001; session × virus: F6,84 < 1). (d, e) Responses during first (reinforcement: F1,14 = 8.49, p = 0.011; reinforcement × virus: F1,14 = 1.59, p = 0.228) and second choice tests (reinforcement: F1,14 = 26.9, p < 0.001; reinforcement × virus: F1,14 < 1). Choice tests were performed on sequential days. Data presented as individual points or mean ± S.E.M. *p < 0.05 (main effect). n = 8 GFP, 8 hM4Di mice. Correspondence to main figures noted. Analyses were performed by ANOVA (2-sided) with repeating measures when appropriate; no adjustments for multiple comparisons required.
Extended Data Fig. 4 Responding during non-reinforced sessions did not differ between groups prior to choice tests.
All non-reinforced sessions were performed drug- and manipulation-free. (a) BLA→OFC inactivation (memory encoding): test 1 (time: F4,108 = 16.5, p < 0.001; time × CNO: F8,108 < 1), test 2 (time: F4,108 = 17.9, p < 0.001; time × CNO: F8,108 < 1), or test 3 (time: F4,108 = 16.2, p < 0.001; time × CNO: F8,108 < 1). (b) BLA→OFC inactivation (delayed memory encoding): test 1 (time: F4,56 = 29.9, p < 0.001; time × CNO: F4,56 < 1) or test 2 (time: F4,56 = 35.2, p < 0.001; time × CNO: F4,56 = 1.63, p = 0.179). (c) BLA→OFC inactivation (memory retrieval): test 1 (time: F4,64 = 12.2, p < 0.001; time × CNO: F4,64 < 1) or test 2 (time: F4,64 = 7.17, p < 0.001; time × CNO: F4,64 < 1). (d) BLA→OFC stimulation: test 1 (time: F4,112 = 16.3, p < 0.001; time × cocaine: F4,112 < 1; time × CNO: F4,112 = 1.18, p = 0.324; time × cocaine × CNO: F4,112 < 1), test 2 (time: F4,112 = 47.0, p < 0.001; time × cocaine: F4,112 = 2.61, p = 0.056; time × CNO: F4,112 = 2.19, p = 0.075; time × cocaine × CNO: F4,112 < 1), or test 3 (time: F4,112 = 55.2, p < 0.001; time × cocaine: F4,112 = 1.27, p = 0.284; time × CNO: F4,112 < 1; time × cocaine × CNO: F4,112 = 1.74, p = 0.147). (e) OFC→DMS inactivation: test 1 (time: F4,72 = 8.30, p < 0.001; time × CNO: F4, 72 < 1) or test 2 (time: F4,72 = 40.5, p < 0.001; time × CNO: F4,72 < 1). (f) OFC→BLA inactivation: test 1 (time: F4,56 = 20.8, p < 0.001; time × CNO: F4,56 < 1) or test 2 (time: F4,56 = 27.1, p < 0.001; time × CNO: F4,56 = 1.62, p = 0.183). (g-h) OFC memory trace inactivation: novel (time: F4,84 = 26.1, p < 0.001; time × 4OHT: F4,84 < 1) or familiar reinforcement conditions (time: F4,72 = 19.3, p < 0.001; time × 4OHT: F4,72 < 1). (i-j) BDNF-dependent circuit function: BLA-OFC (time: F4,136 = 61.6, p < 0.001; time × lateralization: F8,136 < 1) or OFC-DMS disconnections (time: F4,92 = 31.7, p < 0.001; time × lateralization: F8,92 < 1). Data presented as mean ± S.E.M. Correspondence to main figures noted.
Extended Data Fig. 5 Extended interval training prompts inflexible choice behavior.
(a) Responses across training (F14,196 = 16.9, p < 0.001). (b) Choice test responses (t14 < 1). Data presented as individual points or mean ± S.E.M. n = 15 mice. Analyses were performed by ANOVA with repeating measures, and paired t-test (2-sided).
Extended Data Fig. 6 Correlations between choice behavior and relative experience frequency of reinforced vs. non-reinforced nose pokes.
Correlation between individual choice test preference ratios (reinforced / non-reinforced) and the standard contingency measure (ΔP; see Methods) for each 25-minute non-reinforced session. (a) BLA→OFC inactivation: memory encoding (FR1: F1,28 < 1; RI30: F1,28 < 1; RI60: F1,28 = 2.40, p = 0.132), (b) delayed memory encoding (all F1,14 < 1), or (c) memory retrieval (all F1,16 < 1). (d) BLA→OFC stimulation (all F1,30 < 1). (e) OFC→DMS inactivation (all F1,18 < 1). (f) OFC→BLA inactivation (all F1,14 < 1). (g) Correlation coefficients (Pearson’s r) between session ΔP and choice test preference ratios for all experiments in panels a-f (in order). Data presented as individual points or group means. 95% confidence interval (grey shading). Correspondence to main figures noted.
Extended Data Fig. 7 Chemogenetic inactivation of OFC→DMS projections disrupts memory retrieval independent of repeated testing.
(a) Combinatorial viral targeting of OFC→DMS projections. (b) Timing of CNO administration for OFC→DMS projection inactivation during memory retrieval. (c) Responses across training (session: F6,48 = 33.3, p < 0.001; session × CNO: F6,48 < 1). (d) Choice test responses (reinforcement: F1,14 = 15.2, p = 0.002; reinforcement × CNO: F1,14 = 6.74, p = 0.021). Data resented as mean ± S.E.M. *p < 0.05 (post-hoc). n = 8 veh, 8 CNO mice. Experiments were replicated at least once, with concordant results.
Extended Data Fig. 8 Size of chemogenetically inactivated OFC neuronal ensembles does not predict choice behavior.
(a, b) Correlation between number of chemogenetically inactivated OFC neurons and choice test preference ratios (reinforced / non-reinforced) for OFC ensembles labelled following exposure to novel (F1,10 < 1) or familiar reinforcement conditions (F1,8 < 1). Data presented as individual points. 95% confidence interval (shading). Centre lines indicate regression.
Extended Data Fig. 9 Additional dendritic spine parameters among BLA→OFC→DMS relay neurons.
(a) Location of all sampled dendrites from trained (T; filled circles) and yoked (∅; open circles) mice by anterior-posterior (A-P) distance from bregma. (b, c) Dendritic spine density across A-P extent of the ventrolateral OFC for yoked (F1,70 < 1) and trained mice (F1,70 < 1). Centre lines indicate regression. (d–f) Left panels. Dendrite diameter (cocaine: F1,20 < 1; training: F1,20 < 1; cocaine × training: F1,20 < 1), dendritic spine length (cocaine: F1,20 = 3.80, p = 0.053; training: F1,20 = 1.47, p = 0.227; cocaine × training: F1,20 < 1) and dendritic spine diameter (cocaine: F1,20 < 1; training: F1,20 < 1; cocaine × training: F1,20 < 1). Right panels. Percent change (trained mouse vs. yoked cage mate) in dendrite diameter (t10 < 1), dendritic spine length (t10 < 1), and dendritic spine diameter (t10 < 1). Data presented as individual points (solid = per animal; transparent=per dendrite). #p = 0.053 (main effect). n = 6 sal ø, 6 coc ø, 6 sal T, 6 coc T mice.
Extended Data Fig. 10 Correlations between BLA→OFC→DMS circuit-defined dendritic spine plasticity and choice behavior.
Correlation between individual choice test preference ratios (reinforced / non-reinforced) and dendritic spine parameters from Fig.6. (a–d) Dendritic spine density for all spines (F1,10 = 5.12, p = 0.047), and by mushroom- (F1,10 = 5.71, p = 0.038), thin- (F1,10 = 1.14, p = 0.311), and stubby-type spines (F1,10 < 1). (e–h) Percent change (trained mouse vs. yoked [∅] cage mate) in dendritic spine density for all spines (F1,10 = 1.43, p = 0.259), and by mushroom- (F1,10 < 1), thin- (F1,10 = 8.79, p = 0.014), and stubby-type spines (F1,10 = 1.10, p = 0.319). (i-j) Mushroom-to-thin spine-type ratio (F1,10 = 4.88, p = 0.052). Percent change (F1,10 = 4.03, p = 0.073). (k, l) Head volume of mushroom-type spines (F1,10 < 1). Percent change (F1,10 = 8.82, p = 0.014). Data presented as individual points. 95% confidence interval (grey shading). *p < 0.05. †p = 0.052. #p = 0.073. Panels g and l reproduced in Fig.6. Centre lines indicate regression.
Supplementary information
Supplementary Information
Five figures and seven tables.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, D.C., Dighe, N.M., Barbee, B.R. et al. A molecularly integrated amygdalo-fronto-striatal network coordinates flexible learning and memory. Nat Neurosci 25, 1213–1224 (2022). https://doi.org/10.1038/s41593-022-01148-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01148-9
This article is cited by
-
Social experience in adolescence shapes prefrontal cortex structure and function in adulthood
Molecular Psychiatry (2024)
-
GluN2B inhibition confers resilience against long-term cocaine-induced neurocognitive sequelae
Neuropsychopharmacology (2023)
-
Orbitofrontal cortex control of striatum leads economic decision-making
Nature Neuroscience (2023)
-
Training-induced circuit-specific excitatory synaptogenesis in mice is required for effort control
Nature Communications (2023)