Abstract
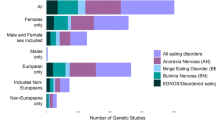
Eating disorders (anorexia nervosa, bulimia nervosa and binge-eating disorder) are a heterogeneous class of complex illnesses marked by weight and appetite dysregulation coupled with distinctive behavioral and psychological features. Our understanding of their genetics and neurobiology is evolving thanks to global cooperation on genome-wide association studies, neuroimaging, and animal models. Until now, however, these approaches have advanced the field in parallel, with inadequate cross-talk. This review covers overlapping advances in these key domains and encourages greater integration of hypotheses and findings to create a more unified science of eating disorders. We highlight ongoing and future work designed to identify implicated biological pathways that will inform staging models based on biology as well as targeted prevention and tailored intervention, and will galvanize interest in the development of pharmacologic agents that target the core biology of the illnesses, for which we currently have few effective pharmacotherapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th edn) (American Psychiatric Publishing, 2013).
Erskine, H. E., Whiteford, H. A. & Pike, K. M. The global burden of eating disorders. Curr. Opin. Psychiatry 29, 346–353 (2016).
Santomauro, D. F. et al. The hidden burden of eating disorders: an extension of estimates from the Global Burden of Disease Study 2019. Lancet Psychiatry 8, 320–328 (2021).
Schaumberg, K. et al. Patterns of diagnostic flux in eating disorders: a longitudinal population study in Sweden. Psychol. Med. 49, 432–450 (2019).
Southern, J. et al. Multi-scale computational modelling in biology and physiology. Prog. Biophys. Mol. Biol. 96, 60–89 (2008).
Yilmaz, Z., Hardaway, J. & Bulik, C. Genetics and epigenetics of eating disorders. Adv. Genomics Genet. 5, 131–150 (2015).
Dellava, J. E., Thornton, L. M., Lichtenstein, P., Pedersen, N. L. & Bulik, C. M. Impact of broadening definitions of anorexia nervosa on sample characteristics. J. Psychiatr. Res. 45, 691–698 (2011).
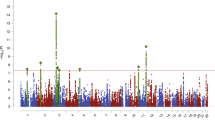
Watson, H. et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51, 1207–1214 (2019).
Duncan, L. et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am. J. Psychiatry 174, 850–858 (2017).
Thornton, L. et al. The Anorexia Nervosa Genetics Initiative: overview and methods. Contemp. Clin. Trials 74, 61–69 (2018).
Song, W., Wang, W., Yu, S. & Lin, G. N. Dissection of the genetic association between anorexia nervosa and obsessive–compulsive disorder at the network and cellular levels. Genes 12, 491 (2021).
Munn-Chernoff, M. A. et al. Shared genetic risk between eating disorder- and substance-use-related phenotypes: evidence from genome-wide association studies. Addict. Biol. 26, e12880 (2021).
Kaye, W. et al. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatry 161, 2215–2221 (2004).
Cederlöf, M. et al. Etiological overlap between obsessive–compulsive disorder and anorexia nervosa: a longitudinal cohort, family and twin study. World Psychiatry 14, 333–338 (2015).
Thornton, L. M., Welch, E., Munn-Chernoff, M. A., Lichtenstein, P. & Bulik, C. M. Anorexia nervosa, major depression, and suicide attempts: shared genetic factors. Suicide Life Threat. Behav. 46, 525–534 (2016).
Dellava, J., Kendler, K. & Neale, M. Generalized anxiety disorder and anorexia nervosa: evidence of shared genetic variation. Depress. Anxiety 28, 728–733 (2011).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Watson, H. J. et al. Common genetic variation and age at onset of anorexia nervosa. Biol. Psychiatry https://doi.org/10.1016/j.bpsgos.2021.09.001 (2021).
Solmi, F., Mascarell, M. C., Zammit, S., Kirkbride, J. B. & Lewis, G. Polygenic risk for schizophrenia, disordered eating behaviours and body mass index in adolescents. Br. J. Psychiatry 215, 428–433 (2019).
Yilmaz, Z., Gottfredson, N., Zerwas, S., Bulik, C. & Micali, N. Developmental premorbid body mass index trajectories of adolescents with eating disorders in a longitudinal population cohort. J. Am. Acad. Child Adolesc. Psychiatry 58, 191–199 (2019).
Abdulkadir, M. et al. Polygenic score for body mass index is associated with disordered eating in a general population cohort. J. Clin. Med. 9, 1187 (2020).
Hübel, C. et al. One size does not fit all. Genomics differentiates among anorexia nervosa, bulimia nervosa, and binge-eating disorder. Int. J. Eat. Disord. 54, 785–793 (2021).
Murray, G. K. et al. Could polygenic risk scores be useful in psychiatry?: a review. JAMA Psychiatry 78, 210–219 (2020).
Wray, N. et al. Research review: polygenic methods and their application to psychiatric traits. J. Child Psychol. Psychiatry 55, 1068–1087 (2014).
Lee, P. H. et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482 (2019).
Lee, J. E., Namkoong, K. & Jung, Y.-C. Impaired prefrontal cognitive control over interference by food images in binge-eating disorder and bulimia nervosa. Neurosci. Lett. 651, 95–101 (2017).
Yilmaz, Z. et al. Examination of the shared genetic basis of anorexia nervosa and obsessive–compulsive disorder. Mol. Psychiatry 25, 2036–2046 (2020).
Reed, Z. E., Micali, N., Bulik, C. M., Davey Smith, G. & Wade, K. H. Assessing the causal role of adiposity on disordered eating in childhood, adolescence, and adulthood: a Mendelian randomization analysis. Am. J. Clin. Nutr. 106, 764–772 (2017).
Tyrrell, J. et al. Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA 315, 1129–1140 (2016).
Adams, D. M., Reay, W. R., Geaghan, M. P. & Cairns, M. J. Investigating the effect of glycaemic traits on the risk of psychiatric illness using Mendelian randomisation. Neuropsychopharmacology 46, 1093–1102 (2020).
Huckins, L. M. et al. Investigation of common, low-frequency and rare genome-wide variation in anorexia nervosa. Mol. Psychiatry 23, 1169–1180 (2018).
Scott-Van Zeeland, A. A. et al. Evidence for the role of EPHX2 gene variants in anorexia nervosa. Mol. Psychiatry 19, 724–732 (2013).
Lutter, M. et al. Novel and ultra-rare damaging variants in neuropeptide signaling are associated with disordered eating behaviors. PLoS ONE 12, e0181556 (2017).
Cui, H. et al. Eating disorder predisposition is associated with ESRRA and HDAC4 mutations. J. Clin. Invest. 123, 4706–4713 (2013).
Lombardi, L. et al. Anorexia nervosa is associated with Neuronatin variants. Psychiatr. Genet. 29, 103–110 (2019).
Wang, K. et al. A genome-wide association study on common SNPs and rare CNVs in anorexia nervosa. Mol. Psychiatry 16, 949–959 (2011).
Yilmaz, Z. et al. Exploration of large, rare CNVs associated with psychiatric and neurodevelopmental disorders in individuals with anorexia nervosa. Psychiatr. Genet 27, 152–158 (2017).
Boraska, V. et al. A genome-wide association study of anorexia nervosa. Mol. Psychiatry 19, 1085–1094 (2014).
Chang, X. et al. Microduplications at the 15q11.2 BP1–BP2 locus are enriched in patients with anorexia nervosa. J. Psychiatr. Res. 113, 34–38 (2019).
Bulik, C. M. et al. The Eating Disorders Genetics Initiative (EDGI): study protocol. BMC Psychiatry 21, 234 (2021).
Co, M., Anderson, A. G. & Konopka, G. FOXP transcription factors in vertebrate brain development, function, and disorders. Wiley Interdiscip. Rev. Dev. Biol. 9, e375 (2020).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700,000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649 (2018).
Zhang, M. et al. Axonogenesis is coordinated by neuron-specific alternative splicing programming and splicing regulator PTBP2. Neuron 101, 690–706 (2019).
Arends, R. M. et al. Associations between the CADM2 gene, substance use, risky sexual behavior and self-control: a phenome-wide association study. Addict. Biol. 26, e13015 (2021).
Rathjen, T. et al. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat. Neurosci. 20, 1096–1103 (2017).
Gerson, S. L. MGMT: its role in cancer aetiology and cancer therapeutics. Nat. Rev. Cancer 4, 296–307 (2004).
Reid, D. A. et al. Incorporation of a nucleoside analog maps genome repair sites in postmitotic human neurons. Science 372, 91–94 (2021).
Wu, W. et al. Neuronal enhancers are hotspots for DNA single-strand break repair. Nature 593, 440–444 (2021).
Finucane, H. et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 47, 1228–1235 (2015).
Skene, N. G. et al. Genetic identification of brain cell types underlying schizophrenia. Nat. Genet. 50, 825–833 (2018).
Chowdhury, T. G. et al. Voluntary wheel running exercise evoked by food-restriction stress exacerbates weight loss of adolescent female rats but also promotes resilience by enhancing GABAergic inhibition of pyramidal neurons in the dorsal hippocampus. Cereb. Cortex 29, 4035–4049 (2019).
Klenowski, P. M. et al. Prolonged consumption of sucrose in a binge-like manner, alters the morphology of medium spiny neurons in the nucleus accumbens shell. Front. Behav. Neurosci. 10, 54 (2016).
Wang, D. et al. Comprehensive functional genomic resource and integrative model for the human brain. Science 362, eaat8464 (2018).
Rossi, M. A. & Stuber, G. D. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 27, 42–56 (2018).
Frank, G. K., Shott, M. E. & DeGuzman, M. C. Recent advances in understanding anorexia nervosa. F1000Res. 8, 504 (2019).
Kaye, W. H., Wagner, A., Fudge, J. L. & Paulus, M. Neurocircuity of eating disorders. Curr. Top. Behav. Neurosci. 6, 37–57 (2010).
Lammel, S., Lim, B. K. & Malenka, R. C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacol 76, 351–359 (2014).
Cowdrey, F. A., Park, R. J., Harmer, C. J. & McCabe, C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol. Psychiatry 70, 736–743 (2011).
Kaye, W. H. et al. Neural insensitivity to the effects of hunger in women remitted from anorexia nervosa. Am. J. Psychiatry 177, 601–610 (2020).
Bohon, C. & Stice, E. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int. J. Eat. Disord. 44, 585–595 (2011).
Simon, J. J. et al. Neural signature of food reward processing in bulimic-type eating disorders. Soc. Cogn. Affect. Neurosci. 11, 1393–1401 (2016).
Bailer, U. F. et al. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol. Psychiatry 61, 1090–1099 (2007).
Broft, A. et al. Striatal dopamine type 2 receptor availability in anorexia nervosa. Psychiatry Res. 233, 380–387 (2015).
Bailer, U. F. et al. Amphetamine induced dopamine release increases anxiety in individuals recovered from anorexia nervosa. Int. J. Eat. Disord. 45, 263–271 (2012).
Broft, A. et al. Striatal dopamine in bulimia nervosa: a PET imaging study. Int. J. Eat. Disord. 45, 648–656 (2012).
Mihov, Y. et al. Metabotropic glutamate receptor 5 in bulimia nervosa. Sci. Rep. 10, 1–10 (2020).
Frank, G. K., Shott, M. E., Hagman, J. O. & Mittal, V. A. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am. J. Psychiatry 170, 1152–1160 (2013).
Craig, A. D. & Craig, A. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Kerr, K. L. et al. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology 41, 521–528 (2016).
Zucker, N. L. et al. The clinical significance of posterior insular volume in adolescent anorexia nervosa. Psychosom. Med. 79, 1025–1035 (2017).
Berner, L. A. et al. Altered anticipation and processing of aversive interoceptive experience among women remitted from bulimia nervosa. Neuropsychopharmacology 44, 1265–1273 (2019).
Ting, J. T. & Feng, G. Neurobiology of obsessive–compulsive disorder: insights into neural circuitry dysfunction through mouse genetics. Curr. Opin. Neurobiol. 21, 842–848 (2011).
Marsh, R. et al. An fMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am. J. Psychiatry 168, 1210–1220 (2011).
Skunde, M. et al. Neural signature of behavioural inhibition in women with bulimia nervosa. J. Psychiatry Neurosci. 41, E69–E78 (2016).
Wierenga, C. et al. Altered BOLD response during inhibitory and error processing in adolescents with anorexia nervosa. PLoS ONE 9, e92017 (2014).
Oberndorfer, T. A., Kaye, W. H., Simmons, A. N., Strigo, I. A. & Matthews, S. C. Demand‐specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. Int. J. Eat. Disord. 44, 1–8 (2011).
Finch, J. E., Palumbo, I. M., Tobin, K. E. & Latzman, R. D. Structural brain correlates of eating pathology symptom dimensions: a systematic review. Psychiatry Res. Neuroimaging 317, 111379 (2021).
Geisler, D. et al. Altered white matter connectivity in young acutely underweight patients with anorexia nervosa. J. Am. Acad. Child Adolesc. Psychiatry 61, 331–340 (2022).
Goodkind, M. et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315 (2015).
McTeague, L. M. et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am. J. Psychiatry 177, 411–421 (2020).
Shanmugan, S. et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am. J. Psychiatry 173, 517–526 (2016).
Wang, S. et al. Neurobiological commonalities and distinctions among 3 major psychiatric disorders: a graph theoretical analysis of the structural connectome. J. Psychiatry Neurosci. 45, 15–22 (2020).
Xia, M. et al. Shared and distinct functional architectures of brain networks across psychiatric disorders. Schizophr Bull. 45, 450–463 (2019).
Hudson, J. I., Hiripi, E., Pope, H. G. Jr. & Kessler, R. C. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol. Psychiatry 61, 348–358 (2007).
Frank, G. K., Favaro, A., Marsh, R., Ehrlich, S. & Lawson, E. A. Toward valid and reliable brain imaging results in eating disorders. Int. J. Eat. Disord. 51, 250–261 (2018).
Kaufmann, L.-K. et al. Fornix under water? Ventricular enlargement biases forniceal diffusion magnetic resonance imaging indices in anorexia nervosa. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 430–437 (2017).
Gaudio, S., Carducci, F., Piervincenzi, C., Olivo, G. & Schiöth, H. B. Altered thalamo–cortical and occipital–parietal–temporal–frontal white matter connections in patients with anorexia and bulimia nervosa: a systematic review of diffusion tensor imaging studies. J. Psychiatry Neurosci. 44, 324–339 (2019).
Thompson, P. M. et al. ENIGMA and global neuroscience: a decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry 10, 100 (2020).
Gelegen, C. et al. Difference in susceptibility to activity-based anorexia in two inbred strains of mice. Eur. Neuropsychopharmacol. 17, 199–205 (2007).
Gelegen, C. et al. Chromosomal mapping of excessive physical activity in mice in response to a restricted feeding schedule. Eur. Neuropsychopharmacol. 20, 317–326 (2010).
Pjetri, E. et al. Identifying predictors of activity based anorexia susceptibility in diverse genetic rodent populations. PLoS ONE 7, e50453 (2012).
Babbs, R. K. et al. Genetic differences in the behavioral organization of binge eating, conditioned food reward, and compulsive-like eating in C57BL/6J and DBA/2J strains. Physiol. Behav. 197, 51–66 (2018).
Newmyer, B. A., Whindleton, C. M., Srinivasa, N., Jones, M. K. & Scott, M. M. Genetic variation affects binge feeding behavior in female inbred mouse strains. Sci. Rep. 9, 1–10 (2019).
Kirkpatrick, S. L. et al. Cytoplasmic FMR1-interacting protein 2 is a major genetic factor underlying binge eating. Biol. Psychiatry 81, 757–769 (2017).
Babbs, R. K. et al. Cyfip1 haploinsufficiency increases compulsive-like behavior and modulates palatable food intake in mice: dependence on Cyfip2 genetic background, parent of origin and sex. G3 9, 3009–3022 (2019).
Farrell, M. et al. Treatment-resistant psychotic symptoms and the 15q11.2 BP1–BP2 (Burnside–Butler) deletion syndrome: case report and review of the literature. Transl. Psychiatry 10, 1–8 (2020).
Yao, E. J. et al. Systems genetic analysis of binge-like eating in a C57BL/6J × DBA/2J-F2 cross. Genes Brain Behav. e12751 (2021).
Nobis, S. et al. Alterations of proteome, mitochondrial dynamic and autophagy in the hypothalamus during activity-based anorexia. Sci. Rep. 8, 1–15 (2018).
Nobis, S. et al. Colonic mucosal proteome signature reveals reduced energy metabolism and protein synthesis but activated autophagy during anorexia‐induced malnutrition in mice. Proteomics 18, 1700395 (2018).
Breton, J. et al. Proteome modifications of gut microbiota in mice with activity-based anorexia and starvation: role in ATP production. Nutrition 67, 110557 (2019).
Schroeder, M. et al. Placental miR-340 mediates vulnerability to activity based anorexia in mice. Nat. Commun. 9, 1–15 (2018).
He, X., Stefan, M., Terranova, K., Steinglass, J. & Marsh, R. Altered white matter microstructure in adolescents and adults with bulimia nervosa. Neuropsychopharmacology 41, 1841–1848 (2015).
Kaakoush, N. O. et al. Alternating or continuous exposure to cafeteria diet leads to similar shifts in gut microbiota compared to chow diet. Mol. Nutr. Food Res. 61, 1500815 (2017).
Sweeney, P. & Yang, Y. Neural circuit mechanisms underlying emotional regulation of homeostatic feeding. Trends Endocrinol. Metab. 28, 437–448 (2017).
Webber, E. S., Bonci, A. & Krashes, M. J. The elegance of energy balance: insight from circuit‐level manipulations. Synapse 69, 461–474 (2015).
Andermann, M. L. & Lowell, B. B. Toward a wiring diagram understanding of appetite control. Neuron 95, 757–778 (2017).
Welch, A. C. et al. Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice. Mol. Psychiatry 26, 3765–3777 (2019).
Milton, L. K. et al. Suppression of corticostriatal circuit activity improves cognitive flexibility and prevents body weight loss in activity-based anorexia in rats. Biol. Psychiatry 90, 819–828 (2021).
Zhang, J. & Dulawa, S. C. The utility of animal models for studying the metabo-psychiatric origins of anorexia nervosa. Front. Psychiatry 12, 711181 (2021).
Foldi, C. J., Milton, L. K. & Oldfield, B. J. The role of mesolimbic reward neurocircuitry in prevention and rescue of the activity-based anorexia phenotype in rats. Neuropsychopharmacology 42, 2292–2300 (2017).
Hebebrand, J., Muller, T., Holtkamp, K. & Herpertz-Dahlmann, B. The role of leptin in anorexia nervosa: clinical implications. Mol. Psychiatry 12, 23–35 (2007).
Verhagen, L. A., Luijendijk, M. C. & Adan, R. A. Leptin reduces hyperactivity in an animal model for anorexia nervosa via the ventral tegmental area. Eur. Neuropsychopharmacol. 21, 274–281 (2011).
Antel, J. et al. Rapid amelioration of anorexia nervosa in a male adolescent during metreleptin treatment including recovery from hypogonadotropic hypogonadism. Eur. Child Adolesc. Psychiatry https://doi.org/10.1007/s00787-021-01778-7 (2021).
Burghardt, P. R. et al. Leptin regulates dopamine responses to sustained stress in humans. J. Neurosci. 32, 15369–15376 (2012).
Wu, Q., Han, Y. & Tong, Q. in Neural Regulation of Metabolism (eds. Wu, Q. & Zheng, R.) 211–233 (Springer, 2018).
Xu, P. et al. Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol. Psychiatry 81, 737–747 (2017).
Marino, R. A. M. et al. Control of food approach and eating by a GABAergic projection from lateral hypothalamus to dorsal pons. Proc. Natl Acad. Sci. USA 117, 8611–8615 (2020).
Wu, H. et al. Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proc. Natl Acad. Sci. USA 115, 192–197 (2018).
Halpern, C. H. et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J. Neurosci. 33, 7122–7129 (2013).
London, T. D. et al. Coordinated ramping of dorsal striatal pathways preceding food approach and consumption. J. Neurosci. 38, 3547–3558 (2018).
Livneh, Y. et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546, 611–616 (2017).
Kusumoto-Yoshida, I., Liu, H., Chen, B. T., Fontanini, A. & Bonci, A. Central role for the insular cortex in mediating conditioned responses to anticipatory cues. Proc. Natl Acad. Sci. USA 112, 1190–1195 (2015).
Price, A. E., Stutz, S. J., Hommel, J. D., Anastasio, N. C. & Cunningham, K. A. Anterior insula activity regulates the associated behaviors of high fat food binge intake and cue reactivity in male rats. Appetite 133, 231–239 (2019).
Wu, Y. et al. The anterior insular cortex unilaterally controls feeding in response to aversive visceral stimuli in mice. Nat. Commun. 11, 640 (2020).
Spierling, S. et al. Insula to ventral striatal projections mediate compulsive eating produced by intermittent access to palatable food. Neuropsychopharmacology 45, 579–588 (2020).
Anastasio, N. C. et al. Convergent neural connectivity in motor impulsivity and high-fat food binge-like eating in male Sprague-Dawley rats. Neuropsychopharmacology 44, 1752–1761 (2019).
Newmyer, B. A. et al. VIPergic neurons of the infralimbic and prelimbic cortices control palatable food intake through separate cognitive pathways. JCI Insight 5, e126283 (2019).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Maussion, G., Demirova, I., Gorwood, P. & Ramoz, N. Induced pluripotent stem cells: new tools for investigating molecular mechanisms in anorexia nervosa. Front. Nutr. 6, 118 (2019).
Breen, G. et al. Translating genome-wide association findings into new therapeutics for psychiatry. Nat. Neurosci. 19, 1392–1396 (2016).
Galmiche, M., Déchelotte, P., Lambert, G. & Tavolacci, M. P. Prevalence of eating disorders over the 2000–2018 period: a systematic literature review. Am. J. Clin. Nutr. 109, 1402–1413 (2019).
Papadopoulos, F., Ekbom, A., Brandt, L. & Ekselius, L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br. J. Psychiatry 194, 10–17 (2009).
Yao, S. et al. Familial liability for eating disorders and suicide attempts: evidence from a population registry in Sweden. JAMA Psychiatry 73, 284–291 (2016).
Fichter, M. M., Quadflieg, N., Crosby, R. D. & Koch, S. Long-term outcome of anorexia nervosa: results from a large clinical longitudinal study. Int J. Eat. Disord. 50, 1018–1030 (2017).
Berkman, N. et al. Management of eating disorders. Evid. Rep. Technol. Assess. 135, 1–166 (2006).
Keski-Rahkonen, A. et al. Epidemiology and course of anorexia nervosa in the community. Am. J. Psychiatry 164, 1259–1265 (2007).
Brownley, K. et al. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann. Intern. Med. 165, 409–420 (2016).
Fornaro, M. et al. Lisdexamfetamine in the treatment of moderate-to-severe binge eating disorder in adults: systematic review and exploratory meta-analysis of publicly available placebo-controlled, randomized clinical trials. Neuropsychiatr. Dis. Treat. 12, 1827–1836 (2016).
Hall, J. F., Smith, K., Schnitzer, S. B. & Hanford, P. V. Elevation of activity level in the rat following transition from ad libitum to restricted feeding. J. Comp. Physiol. Psychol. 46, 429–433 (1953).
Schalla, M. A. & Stengel, A. Activity based anorexia as an animal model for anorexia nervosa—a systematic review. Front Nutr. 6, 69 (2019).
Novelle, M. G. & Diéguez, C. Food addiction and binge eating: lessons learned from animal models. Nutrients 10, 71 (2018).
Corwin, R. L. & Babbs, R. K. Rodent models of binge eating: are they models of addiction? ILAR J. 53, 23–34 (2012).
Moore, C. F., Sabino, V., Koob, G. F. & Cottone, P. Pathological overeating: emerging evidence for a compulsivity construct. Neuropsychopharmacology 42, 1375–1389 (2017).
Rospond, B., Szpigiel, J., Sadakierska-Chudy, A. & Filip, M. Binge eating in preclinical models. Pharmacol. Rep. 67, 504–512 (2015).
Cottone, P., Sabino, V., Steardo, L. & Zorrilla, E. P. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1066–R1076 (2008).
Hardaway, J. A. et al. Nociceptin receptor antagonist SB 612111 decreases high-fat diet binge eating. Behav. Brain Res. 307, 25–34 (2016).
Acknowledgements
C.M.B. is supported by the National Institute of Mental Health (R01MH120170, R01MH124871, R01MH119084, R01MH118278 and R01MH124871), a Brain and Behavior Research Foundation Distinguished Investigator grant, the Swedish Research Council (Vetenskapsrådet, award no. 538-2013-8864), and the Lundbeck Foundation (grant no. R276-2018-4581). J.R.I.C. and G.B. acknowledge that the paper represents independent research part funded by the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. J.A.H. is supported by K01DK115902. C.D.B. is supported by U01DA050243 and U01DA055299. L.B. is supported by T32MH112485, a Harvard Medical School Livingston Fellowship and the International OCD Foundation. G.B. is also supported by the UK Medical Research Council (MR/V012878/1, MR/V03605X/1 and MR/R024804/1) and Charlotte’s Helix.
Author information
Authors and Affiliations
Contributions
C.M.B., J.R.I.C., J.A.H., L.B., C.D.B., H.W. and G.B. all substantially contributed to the writing of the manuscript, approved the submitted version, are personally accountable for their own contribution, and ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
C.M.B. reports the following competing interests: Shire (grant recipient, Scientific Advisory Board member); Idorsia (consultant); Pearson (author, royalty recipient); Equip Health (Clinical Advisory Board Member). G.B. reports the following competing interests: Otsuka Pharma (advisory board); Illumina (grant recipient, conference sponsorship). The other authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Roger Adan, Sandra Sanchez-Roige, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bulik, C.M., Coleman, J.R.I., Hardaway, J.A. et al. Genetics and neurobiology of eating disorders. Nat Neurosci 25, 543–554 (2022). https://doi.org/10.1038/s41593-022-01071-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01071-z
This article is cited by
-
Unexpected identification of obesity-associated mutations in LEP and MC4R genes in patients with anorexia nervosa
Scientific Reports (2024)
-
The multisensory mind: a systematic review of multisensory integration processing in Anorexia and Bulimia Nervosa
Journal of Eating Disorders (2023)
-
Psilocybin for the treatment of anorexia nervosa
Nature Medicine (2023)