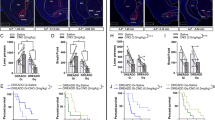
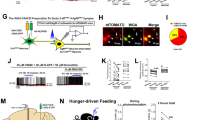
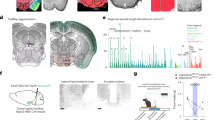
Abstract
Midbrain dopamine (DA) and serotonin (5-HT) neurons regulate motivated behaviors, including feeding, but less is known about how these circuits may interact. In this study, we found that DA neurons in the mouse ventral tegmental area bidirectionally regulate the activity of 5-HT neurons in the dorsal raphe nucleus (DRN), with weaker stimulation causing DRD2-dependent inhibition and overeating, while stronger stimulation causing DRD1-dependent activation and anorexia. Furthermore, in the activity-based anorexia (ABA) paradigm, which is a mouse model mimicking some clinical features of human anorexia nervosa (AN), we observed a DRD2 to DRD1 shift of DA neurotransmission on 5-HTDRN neurons, which causes constant activation of these neurons and contributes to AN-like behaviors. Finally, we found that systemic administration of a DRD1 antagonist can prevent anorexia and weight loss in ABA. Our results revealed regulation of feeding behavior by stimulation strength-dependent interactions between DA and 5-HT neurons, which may contribute to the pathophysiology of AN.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article (and its supplementary information files). Additional data that support the findings of this study are available upon reasonable request from the corresponding authors (Yanlin He and Y.X.). Source data are provided with this paper.
Change history
16 June 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41593-022-01116-3
References
Papadopoulos, F. C., Ekbom, A., Brandt, L. & Ekselius, L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br. J. Psychiatry 194, 10–17 (2009).
Watson, H. J. et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51, 1207–1214 (2019).
Tsai, H. C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Zhou, Q. Y. & Palmiter, R. D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83, 1197–1209 (1995).
Paladini, C. A. & Roeper, J. Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience 282, 109–121 (2014).
Richfield, E. K., Penney, J. B. & Young, A. B. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30, 767–777 (1989).
Bergen, A. W. et al. Association of multiple DRD2 polymorphisms with anorexia nervosa. Neuropsychopharmacology 30, 1703–1710 (2005).
Peng, S. et al. Dopamine receptor D2 and catechol-O-methyltransferase gene polymorphisms associated with anorexia nervosa in Chinese Han population: DRD2 and COMT gene polymorphisms were associated with AN. Neurosci. Lett. 616, 147–151 (2016).
Nisoli, E. et al. D2 dopamine receptor (DRD2) gene Taq1A polymorphism and the eating-related psychological traits in eating disorders (anorexia nervosa and bulimia) and obesity. Eat. Weight Disord. 12, 91–96 (2007).
Frieling, H. et al. Epigenetic dysregulation of dopaminergic genes in eating disorders. Int. J. Eat. Disord. 43, 577–583 (2010).
Gervasini, G. et al. Influence of dopamine polymorphisms on the risk for anorexia nervosa and associated psychopathological features. J. Clin. Psychopharmacol. 33, 551–555 (2013).
Walton, E. et al. Exploration of shared genetic architecture between subcortical brain volumes and anorexia nervosa. Mol. Neurobiol. 56, 5146–5156 (2019).
Barbato, G., Fichele, M., Senatore, I., Casiello, M. & Muscettola, G. Increased dopaminergic activity in restricting-type anorexia nervosa. Psychiatry Res. 142, 253–255 (2006).
Kaye, W. H., Frank, G. K. & McConaha, C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology 21, 503–506 (1999).
Lechin, F., van der Dijs, B. & Hernandez-Adrian, G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 565–585 (2006).
He, Y. et al. 5-HT recruits distinct neurocircuits to inhibit hunger-driven and non-hunger-driven feeding. Mol. Psychiatry 26, 7211–7224 (2021).
McGuirk, J., Goodall, E., Silverstone, T. & Willner, P. Differential effects of d-fenfluramine, l-fenfluramine and d-amphetamine on the microstructure of human eating behaviour. Behav. Pharm. 2, 113–119 (1991).
Xu, P. et al. Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol. Psychiatry 81, 737–747 (2017).
Saller, C. F. & Stricker, E. M. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science 192, 385–387 (1976).
Kaye, W. H., Gwirtsman, H. E., George, D. T. & Ebert, M. H. Altered serotonin activity in anorexia nervosa after long-term weight restoration. Does elevated cerebrospinal fluid 5-hydroxyindoleacetic acid level correlate with rigid and obsessive behavior? Arch. Gen. Psychiatry 48, 556–562 (1991).
Bailer, U. F. et al. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol. Psychiatry 61, 1090–1099 (2007).
Galusca, B. et al. Organic background of restrictive-type anorexia nervosa suggested by increased serotonin 1A receptor binding in right frontotemporal cortex of both lean and recovered patients: [18F]MPPF PET scan study. Biol. Psychiatry 64, 1009–1013 (2008).
Frank, G. K. & Kaye, W. H. Positron emission tomography studies in eating disorders: multireceptor brain imaging, correlates with behavior and implications for pharmacotherapy. Nucl. Med. Biol. 32, 755–761 (2005).
Bailer, U. F. et al. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Res. 211, 160–168 (2013).
Ogawa, S. K., Cohen, J. Y., Hwang, D., Uchida, N. & Watabe-Uchida, M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep. 8, 1105–1118 (2014).
Ogawa, S. K. & Watabe-Uchida, M. Organization of dopamine and serotonin system: anatomical and functional mapping of monosynaptic inputs using rabies virus. Pharmacol. Biochem. Behav. 174, 9–22 (2018).
Li, Y. et al. Rostral and caudal ventral tegmental area GABAergic inputs to different dorsal raphe neurons participate in opioid dependence. Neuron 101, 748–761 (2019).
Pollak Dorocic, I. et al. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 83, 663–678 (2014).
Matthews, G. A. et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617–631 (2016).
Leinninger, G. M. et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323 (2011).
Fink, K. B. & Gothert, M. 5-HT receptor regulation of neurotransmitter release. Pharm. Rev. 59, 360–417 (2007).
Szczypka, M. S. et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron 30, 819–828 (2001).
Mequinion, M., Chauveau, C. & Viltart, O. The use of animal models to decipher physiological and neurobiological alterations of anorexia nervosa patients. Front. Endocrinol. (Lausanne) 6, 68 (2015).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Nectow, A. R. et al. Identification of a brainstem circuit controlling feeding. Cell 170, 429–442 (2017).
Hall, J. F. & Hanford, P. V. Activity as a function of a restricted feeding schedule. J. Comp. Physiol. Psychol. 47, 362–363 (1954).
Boakes, R. A., Mills, K. J. & Single, J. P. Sex differences in the relationship between activity and weight loss in the rat. Behav. Neurosci. 113, 1080–1089 (1999).
Attia, E. Anorexia nervosa: current status and future directions. Annu. Rev. Med. 61, 425–435 (2010).
Floresco, S. B., West, A. R., Ash, B., Moore, H. & Grace, A. A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 6, 968–973 (2003).
Grieder, T. E. et al. Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc. Natl Acad. Sci. USA 109, 3101–3106 (2012).
Chaudhury, D. et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536 (2013).
Frank, G. K. et al. The partial dopamine D2 receptor agonist aripiprazole is associated with weight gain in adolescent anorexia nervosa. Int. J. Eat. Disord. 50, 447–450 (2017).
Frank, G. K. W. Pharmacotherapeutic strategies for the treatment of anorexia nervosa—too much for one drug? Expert Opin. Pharmacother. 21, 1045–1058 (2020).
Klenotich, S. J., Ho, E. V., McMurray, M. S., Server, C. H. & Dulawa, S. C. Dopamine D2/3 receptor antagonism reduces activity-based anorexia. Transl. Psychiatry 5, e613 (2015).
Bello, E. P. et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 14, 1033–1038 (2011).
White, W., Beyer, J. D. & White, I. M. Acute withdrawal-related hypophagia elicited by amphetamine is attenuated by pretreatment with selective dopamine D1 or D2 receptor antagonists in rats. Physiol. Behav. 151, 345–354 (2015).
Szczypka, M. S. et al. Feeding behavior in dopamine-deficient mice. Proc. Natl Acad. Sci. USA 96, 12138–12143 (1999).
Qu, N. et al. A POMC-originated circuit regulates stress-induced hypophagia, depression, and anhedonia. Mol. Psychiatry 25, 1006–1021 (2019).
Boekhoudt, L. et al. Does activation of midbrain dopamine neurons promote or reduce feeding? Int J. Obes. (Lond.) 41, 1131–1140 (2017).
Han, Y. et al. A hindbrain dopaminergic neural circuit prevents weight gain by reinforcing food satiation. Sci. Adv. 7, eabf8719 (2021).
Zhu, X., Ottenheimer, D. & DiLeone, R. J. Activity of D1/2 receptor expressing neurons in the nucleus accumbens regulates running, locomotion, and food intake. Front. Behav. Neurosci. 10, 66 (2016).
Welch, A. C. et al. Dopamine D2 receptor overexpression in the nucleus accumbens core induces robust weight loss during scheduled fasting selectively in female mice. Mol. Psychiatry 26, 3765–3777 (2019).
Land, B. B. et al. Medial prefrontal D1 dopamine neurons control food intake. Nat. Neurosci. 17, 248–253 (2014).
Denis, R. G. P. et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 25, 975 (2017).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Sarinana, J., Kitamura, T., Kunzler, P., Sultzman, L. & Tonegawa, S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc. Natl Acad. Sci. USA 111, 8245–8250 (2014).
Carlson, K. S., Whitney, M. S., Gadziola, M. A., Deneris, E. S. & Wesson, D. W. Preservation of essential odor-guided behaviors and odor-based reversal learning after targeting adult brain serotonin synthesis. eNeuro 3, ENEURO.0257-16.2016 (2016).
He, Y. et al. A small potassium current in AgRP/NPY neurons regulates feeding behavior and energy metabolism. Cell Rep. 17, 1807–1818 (2016).
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Kurokawa, K., Mizuno, K. & Ohkuma, S. Dopamine D1 receptor signaling system regulates ryanodine receptor expression in ethanol physical dependence. Alcohol Clin. Exp. Res. 37, 771–783 (2013).
Sutoo, D. & Akiyama, K. Effect of dopamine receptor anatagonists on the calcium-dependent central function that reduces blood pressure in spontaneously hypertensive rats. Neurosci. Lett. 269, 133–136 (1999).
Pardo, M. et al. Peripheral leptin and ghrelin receptors are regulated in a tissue-specific manner in activity-based anorexia. Peptides 31, 1912–1919 (2010).
Galassetti, P. et al. Effect of sex on counterregulatory responses to exercise after antecedent hypoglycemia in type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 287, E16–E24 (2004).
Dubreucq, S. et al. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol. Psychiatry 73, 895–903 (2013).
Aragona, B. J. et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133–139 (2006).
Atasoy, D., Aponte, Y., Su, H. H. & Sternson, S. M. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Li, C. et al. Defined paraventricular hypothalamic populations exhibit differential responses to food contingent on caloric state. Cell Metab. 29, 681–694 (2018).
Li, Y. et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat. Commun. 7, 10503 (2016).
Fu, Y. et al. Gut hormone GIP induces inflammation and insulin resistance in the hypothalamus. Endocrinology 161, bqaa102 (2020).
Sakata, S. et al. Autosomal recessive complete STAT1 deficiency caused by compound heterozygous intronic mutations. Int. Immunol. 30, 663–671 (2020).
Acknowledgements
The investigators were supported by the following grants from the National Institutes of Health: R01DK114279, R01DK109934 and R21NS108091 to Q.T.; R00 DK107008, R01 DK123098 and P30 DK020595 to P.X.; K01DK119471 to C.W.; R01DK109194 and R56DK109194 to Q.W.; P01DK113954, R01DK115761 and R01DK117281 to Y.X.; R01DK120858 to Q.T. and Y.X.; and P20 GM135002 to Y.H. The investigators were also supported by the US Department of Defense (Innovative Grant W81XWH-19-PRMRP-DA to P.X.), Pew Charitable Trust awards (0026188) to Q.W., Baylor Collaborative Faculty Research Investment Program grants to Q.W., USDA/CRIS (51000-064-01S to Y.X. and Q.W.) and the American Diabetes Association (7-13-JF-61 to Q.W., 1-17-PDF-138 to Y.H. and 1-15-BS-184 to Q.T.). The Ad-iN/WED virus was kindly provided by M. Myers (University of Michigan).
Author information
Authors and Affiliations
Contributions
X.C., H.L. and Yanlin He were involved in experimental design, in most of the procedures, data acquisition and analyses and in writing the manuscript. P.X., H.L., B.F. and C.W. assisted in some of the histology and electrophysiology studies. M.Y., Yang He, H.L., C.L., Y.Y., L.T., N.Z., L.W., N.Y., J.H. and Z.Y. assisted in surgical procedures and in production of study mice. Q.W. and Q.T. were involved in study design and in writing the manuscript. Yanlin He and Y.X. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Roger Adan, Mitsuko Watabe-Uchida and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Distribution of Drd1 and Drd2 in 5-HTDRN neurons.
(a) Representative RNAscope microscopic images showing Tph2, Drd1, Drd2 mRNAs, and their co-localizations, in the mDRN and lDRN. Scale bars = 100 µm. (b-c) Quantification of the number of Tph2+ neurons co-expressing Drd1 alone, Drd2 alone or both in the mDRN (b) and lDRN (c). Results are shown as mean ± s.e.m. with individual data points (n = 3 mice per group). lDRN, lateral DRN; mDRN, medial DRN. (d) Typical action potential traces of 5-HTDRN neurons in response to DA (0.05 µM puff or 10 µM bath perfusion) in the presence of various inhibitors as indicated.
Extended Data Fig. 2 Distribution of tamoxifen-induced Cre activity in DAT-CreER mice and in TPH2-CreER mice.
(a) Representative microscopic images with DAPI counter staining showing the presence or absence of tdTOMATO signals (red) in the VTA, SN, DRN and MRN in DAT-CreER/Rosa26-LSL-tdTOMATO mice. These imaging studies were repeated in 3 mice. (b) Representative microscopic images with DAPI counter staining showing the presence or absence of tdTOMATO signals (red) in the VTA, SN and DRN in TPH2-CreER/Rosa26-LSL-tdTOMATO mice. These imaging studies were repeated in 3 mice. Scale bars are indicated in each panel. DRN, dorsal Raphe nucleus; MRN, median Raphe nucleus; SN, substantia nigra; VTA, ventral tegmental area. Scale bars are indicated in each panel.
Extended Data Fig. 3 Bidirectional effects of the DAVTA→DRN circuit on feeding.
(a-b) Representative images showing EYFP-labelled cell bodies and fibers within the VTA (a) and EYFP-labelled fibers in the DRN (b, repeated in 3 mice. (c) Typical action potential traces of ChR2-expressing DAVTA neurons in response to 2 or 20 Hz photostimulation (repeated in 3 mice). (d) Left panel: refeeding in each of 5-min blocks when 2 Hz blue light was turned on or off. Right panel: total refeeding during the 15-min periods. Results are shown as mean ± s.e.m., *P < 0.05 in two-sided unpaired t-tests (n = 6 or 7 mice per group). (e) Left panel: refeeding in each of 5-min blocks when 20 Hz blue light was turned on or off. Right panel: total refeeding during the 15-min periods. Results are shown as mean ± s.e.m., *P < 0.05 at each 5-min block in two-sided unpaired t-tests (n = 6 or 7 mice per group). (f) Effects of 5 Hz photostimulation of the DAVTA→DRN projections on refeeding. Results are shown as mean ± s.e.m. with individual data points. n = 7 mice. (g) Strategy to activate the DAVTA→DRN projections and simultaneously inhibit 5-HTDRN neurons. (h) Effects of 20 Hz photostimulation on refeeding after i.p. injections of saline or CNO. Results are shown as individual data points. *P < 0.05 in two-sided paired t-tests (n = 8 mice). (i) Food intake measured during the 30-min valence tests. Results are shown as mean ± s.e.m. with individual data points. *P < 0.05 in two-sided paired t-tests (n = 8 mice). (j) Strategy to activate the DAVTA→NAc projections. (k) Effects of 2, 5 and 20 Hz photostimulation of the DAVTA→NAc projections on refeeding. Results are shown as mean ± s.e.m. with individual data points (n = 6 mice). (l-n) Time spent in each chamber with or without blue light stimulation coupled to Chamber 2. Results are shown as individual data points. *P < 0.05 in two-sided unpaired t-tests (n = 5 mice per group).
Extended Data Fig. 4 DRN-projecting DAVTA neurons are activated during activity-based anorexia.
(a) Representative images showing TH-positive neurons within the VTA labelled by GCaMP6. Scale bars are indicated in each panel. Data from 3 different mice were quantified. VTA, ventral tegmental area. (b) Averaged GCaMP6 and isosbestic signals in DRN-projecting DAVTA neurons associated with eating bouts in naïve and ABA mice. Results are shown as mean ± s.e.m. (n = 4 mice per group). (c) Area under the curves (10 seconds after eating bouts) of the GCaMP6 signals in (b). Results are shown as individual data points (n = 4 mice per group). (d) Representative images showing tdTOMATO-positive neurons within the VTA labelled by Green RetroBeads. Scale bars are indicated in each panel. Data from 3 different mice were quantified. VTA, ventral tegmental area. (e) The linear regression curve of cumulative chow intake and DA levels in the DRN during refeeding. Results are shown as individual data points with the linear regression curve plotted. (f) Averaged GRAB_DA1h and isosbestic signals in the DRN associated with eating bouts in naïve and ABA mice. Results are shown as mean ± s.e.m. (n = 4 mice per group). (g) Area under the curves (10 seconds after eating bouts) of the GRAB_DA1h signals in (F). Results are shown as individual data points (n = 4 mice per group).
Extended Data Fig. 5 DRN-projecting DAVTA neurons mediate activity-based anorexia.
(a) Left: a representative image showing TH-positive neurons (green) within the VTA partially co-localize with mCherry (red). Right: a representative image showing mCherry-labelled collateral projections to the NAc. Scale bars are indicated in each panel. NAc, nucleus accumbens; VTA, ventral tegmental area. (b) Left: 30.76% of DAVTA neurons (labelled by TH immunoreactivity) are positive for mCherry; right: 92.36% of mCherry-labelled neurons are positive for TH. Results were quantified from 3 different mice. (c) Typical action potential traces of DRN-projecting DAVTA neurons expressing hM4Di in response to 10 µM CNO. (d-e) Firing frequency (d) and resting membrane potential (e) of DRN-projecting DAVTA neurons in response to 10 µM CNO followed by a wash. Results are shown as individual data points. *P < 0.05 in one-way ANOVA analyses followed by Dunnett’s post hoc test (n = 15 neurons from 3 mice per group). (f) Baseline body weight on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points (n = 7 mice per group).
Extended Data Fig. 6 5-HTDRN neurons mediate activity-based anorexia.
(a) Representative microscopic images showing expression of mCherry (left), TPH2 (middle) and merge (right) in the DRN of TPH2-CreER mice receiving Cre-dependent AAV expressing hM4Di-mCherry in the DRN. Scale bars = 100 µm. Aq, aqueduct; DRN, dorsal Raphe nucleus. (b) Typical action potential traces of 5-HTDRN neurons expressing hM4Di in response to 10 µM CNO. (c-d) Firing frequency (c) and resting membrane potential (d) of 5-HTDRN neurons in response to 10 µM CNO followed by a wash. Results are shown as individual data points. *P < 0.05 in one-way ANOVA analyses followed by Dunnett’s post hoc test (n = 13 neurons from 3 mice per group). (e) Baseline body weight on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points (n = 7 mice per group). (f) Representative microscopic images showing TPH2 immunoreactivity in the DRN of control and TPH2DRN-KO mice. Scale bars = 100 µm. (g) Baseline body weight on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points (n = 8 mice per group).
Extended Data Fig. 7 DRD1 in 5-HTDRN neurons mediates activity-based anorexia.
(a) Baseline body weight of control or DRD1DRN-KO mice on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points. *P < 0.05 in two-sided unpaired t-tests (n = 6 or 9 mice per group). (b) Baseline body weight of control or DRD1TPH2-KO mice on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points (n = 8 or 12 mice per group). (c) RNAscope detecting Tph2 and Drd1 mRNAs in the DRN of control and DRD1TPH2-KO mice. Scale bars = 50 µm. Arrowheads point to double labelled neurons. Quantification of the percentage of Tph2+ neurons co-expressing Drd1 are shown as mean ± s.e.m. with individual data points. *P < 0.05 in unpaired two-sided t-tests (n = 3 mice per group). (d) Firing frequency and resting membrane potential in 5-HTDRN neurons from control or DRD1TPH2-KO mice recorded at the baseline or in response to SKF38393 (1 µM). Results are shown as individual data points. *P < 0.05 between baseline vs. SKF38393; # P < 0.05 between control vs. DRD2TPH2-KO in two-way ANOVA analyses followed by Sidak’s post hoc test (n = 11 or 16 neurons from 3 mice per group). (e) Baseline body weight of saline or SCH23390-treated mice on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points (n = 11 or 12 mice per group).
Extended Data Fig. 8 DRD2 prevents anorexia and weight loss during hyperactivity.
(a) Baseline body weight of control or DRD2DRN-KO mice on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points (n = 7 mice per group). (b) Baseline body weight of control or DRD2TPH2-KO mice on the day before the ABA paradigm started. Results are shown as mean ± s.e.m. with individual data points (n = 6 or 8 mice per group). (c) RNAscope detecting Tph2 and Drd2 mRNAs in the DRN of control and DRD2TPH2-KO mice. Scale bars = 50 µm. Arrowheads point to double labelled neurons. Quantification of the percentage of Tph2+ neurons co-expressing Drd2 are shown as mean ± s.e.m. with individual data points. *P < 0.05 in unpaired two-sided t-tests (n = 3 mice per group). (d) Firing frequency and resting membrane potential in 5-HTDRN neurons from control or DRD2TPH2-KO mice recorded at the baseline or in response to quinpirole (1 µM). Results are shown as individual data points. *P < 0.05 between baseline vs. quinpirole; P = 0.087 between the baseline of control vs. the baseline of DRD2TPH2-KO in two-way ANOVA analyses followed by Sidak’s post hoc test (n = 13 or 15 neurons from 3 mice per group).
Supplementary information
Supplementary Information
Supplementary experimental data, chemical compound characterization data, Supplementary Figs. 1–26, Supplementary Tables 1–3 and copies of 1H and 13C NMR spectra
Source data
Source Data Fig. 1
Statistical source data and electrophysiological traces
Source Data Fig. 1
Raw microscopic image
Source Data Fig. 2
Statistical source data
Source Data Fig. 3
Statistical source data and electrophysiological traces
Source Data Fig. 4
Statistical source data
Source Data Fig. 5
Statistical source data
Source Data Fig. 6
Statistical source data
Source Data Extended Data Fig. 1
Statistical source data and electrophysiological traces
Source Data Extended Data Fig. 1
Raw microscopic image
Source Data Extended Data Fig. 2
Raw microscopic image
Source Data Extended Data Fig. 3
Statistical source data and electrophysiological traces
Source Data Extended Data Fig. 3
Raw microscopic image
Source Data Extended Data Fig. 4
Statistical source data
Source Data Extended Data Fig. 4
Raw microscopic image
Source Data Extended Data Fig. 5
Statistical source data and electrophysiological traces
Source Data Extended Data Fig. 5
Raw microscopic image
Source Data Extended Data Fig. 6
Statistical source data and electrophysiological traces
Source Data Extended Data Fig. 6
Raw microscopic image
Source Data Extended Data Fig. 7
Statistical source data
Source Data Extended Data Fig. 7
Raw microscopic image
Source Data Extended Data Fig. 8
Statistical source data
Source Data Extended Data Fig. 8
Raw microscopic image
Rights and permissions
About this article
Cite this article
Cai, X., Liu, H., Feng, B. et al. A D2 to D1 shift in dopaminergic inputs to midbrain 5-HT neurons causes anorexia in mice. Nat Neurosci 25, 646–658 (2022). https://doi.org/10.1038/s41593-022-01062-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-022-01062-0
This article is cited by
-
A modular organic neuromorphic spiking circuit for retina-inspired sensory coding and neurotransmitter-mediated neural pathways
Nature Communications (2024)
-
Unraveling the serotonin saga: from discovery to weight regulation and beyond - a comprehensive scientific review
Cell & Bioscience (2023)
-
AgRP neurons coordinate the mitigation of activity-based anorexia
Molecular Psychiatry (2023)