Abstract
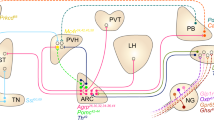
Despite notable genetic influences, obesity mainly results from the overconsumption of food, which arises from the interplay of physiological, cognitive and environmental factors. In patients with obesity, eating is determined more by external cues than by internal physiological needs. However, how environmental context drives non-homeostatic feeding is elusive. Here, we identify a population of somatostatin (TNSST) neurons in the mouse hypothalamic tuberal nucleus that are preferentially activated by palatable food. Activation of TNSST neurons enabled a context to drive non-homeostatic feeding in sated mice and required inputs from the subiculum. Pairing a context with palatable food greatly potentiated synaptic transmission between the subiculum and TNSST neurons and drove non-homeostatic feeding that could be selectively suppressed by inhibiting TNSST neurons or the subiculum but not other major orexigenic neurons. These results reveal how palatable food, through a specific hypothalamic circuit, empowers environmental context to drive non-homeostatic feeding.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are contained in the main text or extended data. Source data can be downloaded at https://doi.org/10.6084/m9.figshare.14532090.v1.
Code availability
Source code can be downloaded at https://doi.org/10.6084/m9.figshare.14532096.v1.
References
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Swinburn, B., Sacks, G. & Ravussin, E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am. J. Clin. Nutr. 90, 1453–1456 (2009).
Schachter, S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science 161, 751–756 (1968).
Woods, S. C. Signals that influence food intake and body weight. Physiol. Behav. 86, 709–716 (2005).
Holland, P. C. & Petrovich, G. D. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol. Behav. 86, 747–761 (2005).
Hill, J. O. & Peters, J. C. Environmental contributions to the obesity epidemic. Science 280, 1371–1374 (1998).
Weingarten, H. P. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 220, 431–433 (1983).
Stern, S. A., Doerig, K. R., Azevedo, E. P., Stoffel, E. & Friedman, J. M. Control of non-homeostatic feeding in sated mice using associative learning of contextual food cues. Mol. Psychiatry https://doi.org/10.1038/s41380-018-0072-y (2018).
Petrovich, G. D., Ross, C. A., Gallagher, M. & Holland, P. C. Learned contextual cue potentiates eating in rats. Physiol. Behav. 90, 362–367 (2007).
de Araujo, I. E., Schatzker, M. & Small, D. M. Rethinking food reward. Annu. Rev. Psychol. 71, 139–164 (2020).
Wise, R. A. Role of brain dopamine in food reward and reinforcement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1149–1158 (2006).
Lutter, M. & Nestler, E. J. Homeostatic and hedonic signals interact in the regulation of food intake. J. Nutr. 139, 629–632 (2009).
Berthoud, H. R., Munzberg, H. & Morrison, C. D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152, 1728–1738 (2017).
Petrovich, G. D. Forebrain networks and the control of feeding by environmental learned cues. Physiol. Behav. 121, 10–18 (2013).
Sternson, S. M. & Eiselt, A. K. Three pillars for the neural control of appetite. Annu. Rev. Physiol. 79, 401–423 (2017).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Jennings, J. H. et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527 (2015).
Zhang, X. & van den Pol, A. N. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356, 853–859 (2017).
Luo, S. X. et al. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361, 76–81 (2018).
Chen, Y., Lin, Y. C., Kuo, T. W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22, 460–469 (2019).
Sternson, S. M. & Roth, B. L. Chemogenetic tools to interrogate brain functions. Annu. Rev. Neurosci. 37, 387–407 (2014).
Mickelsen, L. E. et al. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 22, 642–656 (2019).
Callaway, E. M. & Luo, L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J. Neurosci. 35, 8979–8985 (2015).
Torromino, G. et al. Offline ventral subiculum–ventral striatum serial communication is required for spatial memory consolidation. Nat. Commun. 10, 5721 (2019).
Bienkowski, M. S. et al. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat. Neurosci. 21, 1628–1643 (2018).
Vardy, E. et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86, 936–946 (2015).
Mu, M. D. et al. A limbic circuitry involved in emotional stress-induced grooming. Nat. Commun. 11, 2261 (2020).
Velazquez-Sanchez, C. et al. Seeking behavior, place conditioning, and resistance to conditioned suppression of feeding in rats intermittently exposed to palatable food. Behav. Neurosci. 129, 219–224 (2015).
Spyraki, C., Fibiger, H. C. & Phillips, A. G. Attenuation by haloperidol of place preference conditioning using food reinforcement. Psychopharmacology (Berl.) 77, 379–382 (1982).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665–678.e23 (2018).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Zhu, C. et al. Profound and redundant functions of arcuate neurons in obesity development. Nat. Metab. 2, 763–774 (2020).
Zhao, Z. D. et al. Zona incerta GABAergic neurons integrate prey-related sensory signals and induce an appetitive drive to promote hunting. Nat. Neurosci. 22, 921–932 (2019).
Li, Y. et al. Hypothalamic circuits for predation and evasion. Neuron 97, 911–924.e5 (2018).
Shang, C. et al. A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nat. Neurosci. 22, 909–920 (2019).
Jais, A. et al. PNOCARC neurons promote hyperphagia and obesity upon high-fat-diet feeding. Neuron 106, 1009–1025.e10 (2020).
Cole, S., Hobin, M. P. & Petrovich, G. D. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience 286, 187–202 (2015).
Walker, A. K., Ibia, I. E. & Zigman, J. M. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiol. Behav. 108, 34–43 (2012).
Petrovich, G. D., Ross, C. A., Holland, P. C. & Gallagher, M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J. Neurosci. 27, 6436–6441 (2007).
Euston, D. R., Gruber, A. J. & McNaughton, B. L. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012).
Gogolla, N. The insular cortex. Curr. Biol. 27, R580–R586 (2017).
Wardle, J. Conditioning processes and cue exposure in the modification of excessive eating. Addict. Behav. 15, 387–393 (1990).
Jansen, A., Schyns, G., Bongers, P. & van den Akker, K. From lab to clinic: extinction of cued cravings to reduce overeating. Physiol. Behav. 162, 174–180 (2016).
Garfield, A. S. et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 19, 1628–1635 (2016).
Seeley, R. J., Payne, C. J. & Woods, S. C. Neuropeptide Y fails to increase intraoral intake in rats. Am. J. Physiol. 268, R423–R427 (1995).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Tong, Q., Ye, C. P., Jones, J. E., Elmquist, J. K. & Lowell, B. B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000 (2008).
Harris, J. A. et al. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front. Neural Circuits 8, 76 (2014).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e22 (2018).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
He, M. et al. Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron 91, 1228–1243 (2016).
Resendez, S. L. et al. Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat. Protoc. 11, 566–597 (2016).
Azevedo, E. P. et al. A role of Drd2 hippocampal neurons in context-dependent food intake. Neuron 102, 873–886.e5 (2019).
Tellez, L. A. et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat. Neurosci. 19, 465–470 (2016).
Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017).
Zhou, P. et al. Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. eLife https://doi.org/10.7554/eLife.28728 (2018).
Zhang, Z. et al. Activation of the dopaminergic pathway from VTA to the medial olfactory tubercle generates odor-preference and reward. eLife https://doi.org/10.7554/eLife.25423 (2017).
Acknowledgements
We thank X. Xu, X. K. Chen and the Neuroscience Pioneer Club for critical reading and discussion of the manuscript. We thank J. Huang for providing the SST-Flp mice and B. Li for providing the AAV-fDIO-Cre plasmid. This work was supported by an A*STAR Investigatorship provided by the Biomedical Research Council (BMRC) of A*STAR (1530700142 to Y.F.), the Singapore Ministry of Education (MOE2017-T3-1-002 to G.J.A.) and the National Natural Science Foundation of China (31900708 to Q. Li). E.S. is supported by a SINGA graduate scholarship from the A*STAR Graduate Academy.
Author information
Authors and Affiliations
Contributions
Y.F. conceived the project and wrote the manuscript. H.M., E.S. and Y.F. designed and implemented the study. M.G., C.-Y.L., X.Y.Y., S.J. and G.J.A. contributed the electrophysiological recordings. Q. Li, Q. Liu and F.X. contributed the fiberphotometry recordings. M.W. assisted with optical systems. A.L. contributed anatomical tracing. S.X.L. helped establish the initial CFC paradigm. All authors discussed the results, commented on and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Ivan de Araujo, Michael Krashes, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
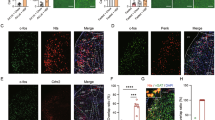
Extended Data Fig. 1 TNSST neuronal activity is increased with palatable diets.
a, Representative images of FISH for VGAT (red) and cFos (green) mRNA and quantification of c-Fos-positive LHVGAT neurons in mice after fasting (7 sections from n = 3), or consuming chow (8 sections from n = 4) or HFD (8 sections from n = 4), or chocolate pellets (14 sections from n = 3) for 1 hour post-fasting (one-way ANOVA with Tukey’s multiple comparison test F(3, 33) = 0.7216; p(fasted vs chow)= 0.9183; p(fasted vs high fat)= 0.6731; p(fasted vs choc)= 0.9787). b, Representative images of FISH of VGAT (red) and cFos (green) mRNA and quantification of c-Fos-positive ZIVGAT neurons after fasting (7 sections from n = 3), or consuming chow (8 sections from n = 4 mice) or HFD (7 sections from n = 4) or chocolate pellets (14 sections from n = 3) for 1 hour post-fasting (F(3, 32) = 1.140; p(fasted vs chow)= 0.2928; p(fasted vs high fat)= 0.9994; p(fasted vs choc)= 0.8855). Scale bars (a and b), 200 m. c, Quantification of cFos-positive TNSST neurons rostro-caudally in mice after fasting (18 sections from n = 4), or consuming normal chow (20 sections from n = 5) or HFD (19 sections from n = 6) for 1 hour post-fasting (Anterior: F(2, 12) = 11.86, p(fasted vs chow)= 0.2949, p(fasted vs high fat)= 0.018, p(chow vs high fat)= 0.0011; Medial: F(2,25)= 26.33, p(fasted vs chow)= 0.1555, p(fasted vs high fat)= 0.0001, p(chow vs high fat)<0.0001; Posterior: F(2,11)=29.49, p(fasted vs chow)= 0.1089, p(fasted vs high fat)= 0.0013, p(chow vs high fat)<0.0001, **p < 0.01). d, Quantification of Ghsr+ and Ghsr- c-Fos+ TNSST neurons along rostro-caudal axis after consuming HFD for 1 hour post- fasting (Ghsr + : F(2,10)=2.101; p = 0.1730; Ghsr-: F(2,10)=2.101; p = 0.1730). e, Quantification of cFos+ TNSST neurons using immunohistochemical staining, with representative coronal sections after fasting (17 sections from n = 3), or consuming normal chow (17 sections from n = 3) or HFD (18 sections from n = 3) for 2 hour post-fasting (F(2,49)=37.57; p(fasted vs chow)= 0.9273; p(fasted vs high fat)<0.0001; p(chow vs high fat)<0.0001, ***p < 0.001). One-way ANOVA with Tukey’s multiple comparison test was used for all panels in this figure. All data in figure are presented as mean values±SEM.
Extended Data Fig. 2 Further characterisation of TNSST neuronal response to palatable diets.
a, Experimental design for fibre photometry experiment. b and c, Population activity of TNSST neurons (mean ± SEM) while approaching chocolate pellets (b) (two-sided paired t-test p = 0.0231), and while consuming chocolate pellets (c) (two-sided paired t-test p = 0.0417). d, Experimental design showing microendoscopic imaging in freely behaving mice in an arena with high fat corner (HFC) and chocolate corner (CC). e, Venn diagram showing overlap between CP-responsive and HFD-responsive TNSST neurons. f, Traces of TNSST activity (mean ± SEM) when entering the HFC or CC corner in the arena. g, Experimental strategy to identify proportion of TNSST neurons responding to both HFD and CP. The schematic shows that TNSST neurons were TRAPed in response to HFD (similarly, the CP-responsive TNSST neurons were TRAPed by changing HFD with CP). h, Images showing TNSST neurons responding to HFD (#), CP(*) or to both (white arrows) when HFD was paired with 4-OHT administration for TRAP. i, Images showing TNSST neurons responding to HFD (*), CP(#) or to both (white arrows) when CP was paired with 4-OHT administration for TRAP. # = YFP + Tdtomato + , * = YFP + c-Fos + , white arrow = YFP + Tdtomato+c-Fos + . Scale bar, 200 µm. j, Illustration showing how above strategy label putative neurons that respond to both HFD and CP. k, Pie graphs showing percentage of TNSST neurons responding to HFD, CP or to both quantified from (h) and (i) n = 3 mice for both pie graphs. All data in figure are presented as mean values±SEM.
Extended Data Fig. 3 TNSST neurons were not activated by post-ingestive calories but their activity is critical for HFD consumption.
a, Experimental design. SST-Cre::Ai14 mice were administered water, sucralose (non-caloric sweet), sucrose, saturated fat, chocolate shake or ensure by feeding needle into the stomach. b, Representative images of c-Fos expression in TNSST neurons after administration of water, sucralose (non-caloric sweet) sucrose, saturated fat, chocolate shake, or ensure into the stomach, from 3 independent experiments. Scale bar, 200 µm. c, Scatter plot showing quantification of c-Fos positive TNSST neurons in mice after administration of water (12 sections from n = 4), sucralose (12 sections from n = 4), sucrose(12 sections from n = 4), saturated fat(20 sections from n = 3), chocolate shake(22 sections from n = 3) or ensure (15 sections from n = 2). (one-way ANOVA F(5,14)= 1.422; p = 0.2761). d, Experimental design with virus injection strategy and arena with high fat diet corner (HFC, yellow), normal chow diet corner (NCC, red), plastic food mimic corner (FMC, violet) and empty corner (EC, green). e and f, Food consumption after TNSST neurons were chemogenetically activated in sated (e) (n = 9; two-sided paired t-test; high fat: p < 0.0001, chow: p = 0.7802, ***p < 0.001) or chemogenetically inhibited in fasted state (f) (n = 9; two-sided paired t-test; high fat: p < 0.0001, chow: p = 0.7458, ***p < 0.001). All data in figure are presented as mean values±SEM.
Extended Data Fig. 4 Control experiments for TNSST or LHVGAT mediated CFC.
a, Virus injection strategy for chemogenetically activating orexigenic neurons in hypothalamus. b, Representative images showing bilateral injections targeting TNSST, LHVGAT and AgRP neurons from at least 3 independent experiments. Scale bar: 500 µm. c, Schematic of CFC paradigm without food (for d and e, food was not available during the conditioning phase of CFC, and food was available on test day), or with wood stick (for f-h, only wood stick was available during the conditioning phase of CFC, and both food and wood stick were available on test day). d and e, Data showing mice did not get conditioned to eat more in the test phase than baseline after activating TNSST (n = 8) (d) (two-sided paired t-test p = 0.781) or LHVGAT (n = 7) (e) (two-sided paired t-test p = 0.3253) neurons during CFC without food. f and g, Data showing mice did not get conditioned to eat more in the test phase than baseline after activating TNSST (n = 6) (f) (paired t-test p = 0.2228), or LHVGAT (n = 6) (g) (paired t-test p = 0.1342) neurons during CFC with only wood stick. h, Data showing activating TNSST (n = 6) neurons did not induce chewing of wood stick during conditioning and mice did not chew wood stick in test phase of CFC, whereas activating LHVGAT (n = 6) neurons induced chewing of wood stick in conditioning phase but not in test phase (LHVGAT: one-way ANOVA with Tukey’s multiple comparison F(2,18)=13.83; p(baseline vs conditioning)= 0.0005, p(baseline vs test)=0.969, p(conditioning vs test)=0.0009). i, Schematic of protocol for evaluating whether the effect on eating specifically depends on the context after TNSST mediated CFC (blue boxes = Phenotyper, orange boxes = Phenotyper with contextual cue, blue circle with red spikes =chemogenetically activating neurons). j, Data showing significant decrease in food intake in the absence of contextual cue on day 5. Food intake was increased on the following day 6 when contextual cue was presented (n = 5; two-sided paired t-test; p(day4 vs day5)= 0.0063, p(day4 vs day6)=0.86, p(day5 vs day6)=0.01). k, Data showing no effect on food intake after activating LHSST neurons (n = 4 mice; Wilcoxon matched-pairs test p = 0.125). l, Representative image showing expression of hM3D in LHSST neurons in SST-cre mice from 3 independent experiments. m, Representative image showing expression of hM3D in DMHSST neurons in SST-cre (left panel) mice and an image of FISH of Vglut 2 and SST mRNA in coronal section of DMH (right panel). n, Data showing no effect on food intake after activation of DMHSST neurons in fed mice during day (n = 8; two-sided paired t-test p = 0.0794, left panel) and after overnight fasting (n = 5; paired t-test p = 0.137, right panel). o, Schematic of protocol for assessing food intake 6 days after TNSST (n = 6) or LHVGAT (n = 4) mediated CFC. p, Data showing food intake in mice conditioned either by activating TNSST neurons (one-way ANOVA with Tukey’s multiple comparison F(6,30)=6.396; p(baseline vs test1)=0.0002; p(baseline vs test2)=0.0018; p(baseline vs test3)=0.0003; p(baseline vs test4)=0.0137; p(baseline vs test5)=0.0064; p(baseline vs test6)=0.0663) or LHVGAT neurons (one-way ANOVA with Tukey’s multiple comparison F(6,18)=3.19; p(baseline vs test1)=0.0213; p(baseline vs test2)=0.0935; p(baseline vs test3)=0.351; p(baseline vs test4)=0.0424; p(baseline vs test5)=0.1061; p(baseline vs test6)=0.0486). All data in figure are presented as mean values±SEM with individual data plots.
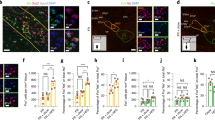
Extended Data Fig. 5 Control experiments for characterizing vSub to hypothalamus projections and their response in CFC.
a, Rabies tracing strategy to identify the inputs from ventral subiculum (vSub) to LHVGAT neurons (n = 3). b, Representative coronal section images showing starter LHVGAT neurons (white arrows) and inputs in the vSub from 3 independent experiments. c, Injection strategy for identifying vSub neurons projecting to lateral hypothalamus and TNSST. Rabies viral tracing was combined with the retrograde tracer cholera toxin B subunit 647 (CTB 647) to identify vSub projection patterns in these two hypothalamic regions. d, Coronal section showing TNSST starter cells in TN (white arrows) and CTB 647 injection (blue) in LH from 3 independent experiments. e, Coronal section showing vSub neurons either labelled by RV-mCherry alone or co-labelled (white arrows) by rabies mCherry and CTB from 3 independent experiments. f, Quantification showing around 50% of TNSST-projecting vSub neurons also project to LH. g, Injection strategy for determining if glutamatergic or GABAergic vSub neurons project to the TN. Vglut-1 cre and VGAT-cre mice were injected with AAV1-CAG-FLEX-GFP and retrograde CTB647 was targeted to TN in the same mice. h, Representative coronal sections showing targeting of CTB647 in TN in Vglut1-cre and VGAT-cre mice from 3 independent experiments. i, Representative coronal sections showing very dense projections in the TN from vSub in Vglut1-cre mice in comparison to VGAT-cre mice from 3 independent experiments. j, Representative coronal sections of vSub showing GFP labelled neurons overlap with the retrograde tracer CTB647 injected in TN of Vglut1-cre mice, however no overlap was seen in VGAT-cre mice. k, Injection strategy for testing whether the connection between vSub and TNSST is glutamatergic or GABAergic. AAV5-hSyn-ChR2-EYFP was injected to vSub of SST-Cre:Ai14 mice. l, Representative trace showing glutamatergic response was completely blocked upon application of glutamate receptor antagonist CNQX + APV from 3 independent experiments. m, Quantification showing complete abolition of amplitude response in all cells after CNQX + APV treatment (n = 8 cells from 3 mice; Mann-Whitney U test; p = 0.0002, *** p < 0.001). n, Schematic for Chr2 intensities quantification around patched cells after electrophysiological recordings. o, Data showing correlation between AMPA magnitude at -70mV and mean intensity of YFP expression. p, Scatter plot showing no difference in Chr2 expression level between control and conditioned mice (control=21 cells from 4 mice, conditioned=25 cells from 5 mice; two-sided unpaired t-test with Welch’s correction p = 0.1068). q, Data showing significant difference in the EPSCs of the above 21 control cells and 25 conditioned cells whose Chr2 intensity was measured (two sided unpaired t-test with Welch’s correction p = 0.02). r, Table showing number of connected cells between vSub to TNSST in our recordings in control and conditioned mice. Chi-square test showed no significant difference. All data in figure are presented as mean values±SEM.
Extended Data Fig. 6 Inhibition of vSub inputs abolished LHVGAT mediated CFC and activating vSub inputs were not sufficient to induce food intake.
a, Viral injection strategy for inhibiting vSub inputs during test phase. b, Schematic of inhibiting vSub inputs during test phase of CFC paradigm. c, Chemogenetic inhibition of vSub inputs by Salvinorin B on test day 2 reduced the food intake after CFC. Data shown as mean values±SEM (n = 6; one-way ANOVA with Tukey’s multiple comparison, F(1.187, 5.933) =17.63; p(test day1 vs salb)=0.0015; p(test day1 vs dmso)=0.1348; p(salb vs dmso)=0.0103. d, Representative images showing expression of KORD-mCitrine in vSub and hM3D-mcherry in LHVGAT neurons from 3 independent experiments. Scale bar, 500 µm (vSub) and 200 µm (LH). e, Viral injection strategy for activating vSub to TN circuitry. AAVrg-cre virus was injected in TN and cre-dependent AAV-DIO-hM3D-mCherry was injected in vSub. f, Representative coronal section showing expression of hM3D-mcherry in the vSub from 3 independent experiments. Scale bar, 500 µm. g-j, TN-projecting vSub neurons were chemogenetically activated and 3 hrs post injection eating behavior was analyzed. Food intake (g) (two-sided paired t-test p = 0.569), mean eating bout duration (h) (two-sided paired t-test p = 0.969), eating frequency (i) (two-sided paired t-test p = 0.31) per hour, and cumulative eating time (j) (two-sided paired t-test p = 0.59) were measured. No significant difference was observed between saline (black dots) and CNO (red dots) treatments (n = 6) Data shown as mean values±SEM. k and l, Data showing no effect of CNO on food intake in wildtype mice after fast-refeeding (k) (two-sided paired t-test p = 0.1) and during day in fed mice (l) (two-sided paired t-test p = 0.13) (n = 8 mice for both).
Extended Data Fig. 7 Physiological contextual feeding conditioning (pCFC) with different food.
a, pCFC paradigm using normal chow diet or chocolate pellet (CP) (gray boxes = home-cage, orange boxes = context). During the conditioning phase only chocolate pellets (CP) or chow were provided in the conditioning context. b, Data showing increased food intake during test phase only in mice conditioned with CP (n = 5 control, n = 4 chow, n = 7 chocolate pellet) (one-way ANOVA with Tukey’s multiple comparison F(2,13)=43.78; p(control vs conditioned with CP) < 0.0001; p(control vs conditioned with chow)=10.92). c, Heat map of mice location during pCFC showing CP-conditioned mice spent more time in CP corner after pCFC. d, pCFC paradigm using normal chow diet or High fat diet (HFD) (gray boxes = home-cage, orange boxes = context). e, Data showing increased food intake during test phase also in mice conditioned with HFD (n = 7 control, n = 8 HFD) (unpaired t-test; ptest day(control vs conditioned)=0.0043). f, Representative images FISH for VGAT, c-Fos and SST in control and CP-conditioned mice from 3 independent experiments. g, Quantification showing percentage of cFos positive LHVGAT or ZIVGAT neurons in control (n = 4) and conditioned mice (n = 4). No significant difference was observed between control and conditioned mice in c-Fos positive percentage. (Mann-Whitney U test, LHVGAT: p = 0.281; ZIVGAT: p = 0.1188). All data in figure are presented as mean values±SEM.
Extended Data Fig. 8 vSub-LH connection is not potentiated after pCFC and inhibition of vSub inputs to LH has no effect on feeding.
a, Representative images showing expression of AAV5-CamkII-ChR2-EYFP in vSub of control and conditioned VGAT-Cre::Ai14 mice from 3 independent experiments. Scale bar, 500 µm. b, Data showing similar fluorescence intensity of ChR2-EYFP expression in vSub of control and conditioned mice. (Mann-Whitney U test, p = 0.743; 8 slices from control and 9 slices from conditioned from n = 3 mice/group) c, Viral injection strategy for ex-vivo whole cell voltage-clamp recording of light evoked synaptic transmission between vSub and LHVGAT neurons in VGAT-Cre::Ai14 mice after pCFC paradigm. d, Quantification of light evoked EPSC amplitudes after pCFC in control (9 cells from 3 mice) and conditioned mice (9 cells from 4 mice), each dot represents one neuron (two sided unpaired t-test with Welch’s correction p = 0.6572). e, Data showing similar fluorescence intensity of ChR2-EYFP expression around patched neurons in control (n = 5 neurons) and conditioned (n = 7 neurons) mice (two-sided unpaired t-test with Welch’s correction, p = 0.3692). f, Representative images to show AAV-DIO-hM4D-mCherry expression in ArcAgRP, TNSST and LHVGAT in AgRP-Cre, SST-Cre and VGAT-Cre mice respectively from 3 independent experiments. g, Viral injection strategy for inhibiting vSub inputs to LH. AAVrg-cre virus was injected in LH and cre-dependent AAV-DIO-hM4D-mCherry was injected in vSub. vSub neurons were chemogenetically inhibited before providing food. h, Food intake in saline (black) and CNO (red) injected mice in refeeding after overnight fasting (n = 8, two-sided paired t-test, p = 0.7837). i, Representative image showing expression of AAV-DIO-hM4D-mCherry in vSub from 3 independent experiments. All data in figure are presented as mean values±SEM.
Extended Data Fig. 9 Inhibition of vSub inputs to TN has no effect on homeostatic feeding, exploratory and grooming behaviours.
a, Viral injection strategy to inhibit vSub inputs to TN. AAVrg-cre virus was injected in TN and cre-dependent AAV-DIO-hM4D-mCherry was injected in vSub. b-d, Data showing no overall change in food intake during refeeding after overnight fasting (b) (two-sided paired t-test p = 0.9056), and in overnight food intake (c) (two-sided paired t-test p = 0.297) as well when measured 3 hrs after CNO injection at the onset of dark phase (d) (two-sided paired t-test p = 0.6428). e-g, Continuously measured mean eating bout duration (e), eating frequency (f) per hour, and cumulative eating time (g) pattern remained relatively similar as previous days. Thick lines represent mean value, thin gray lines represent individual mouse. Gray arrowheads show saline injection, purple arrowheads show CNO injection in panel. h, Representative heat map of exploration after saline or CNO injection in the open field arena from 3 independent experiments. i-k, Data showing effects on distance travelled (i) (two-sided paired t-test p = 0.107), grooming (j) (two-sided paired t-test p = 0.1083) and rearing (k) (two-sided paired t-test p = 0.031) in the open field. l-o, Elevated plus maze (EPM) analysis also showed no significant changes after inhibiting vSub input to TN. Representative heat map showing exploration in open and closed arms after saline or CNO injection in the EPM from 3 independent experiments (l). Mice showed no difference in time spent in open and closed arms (m) (two-sided unpaired t-test with Welch’s correction, open arm: p = 0.3672; closed arm: p = 0.6741), frequency to enter open and closed arms (n) (two-sided unpaired t-test with Welch’s correction, open arm: p = 0.4582; closed arm: p = 0.6815) and latency to enter open and closed arms (o) (two-sided unpaired t-test with Welch’s correction, open arm: p = 0.8782; closed arm: p = 0.9906) (n = 8 mice for both saline and CNO groups). Box plots show median (centre line) quartiles (boxes) and range (whiskers) in panels m-o. p, Representative images showing CTB 555 and CTB 488 injection sites in LS and TN respectively along with input cells in anterior and posterior vSub from 3 independent experiments.
Extended Data Fig. 10 Food consumption during conditioning phase of CPP.
Averaged food intake after inhibiting hypothalamic orexigenic neurons during conditioning phase of CPP (SST n = 9, AgRP n = 9, VGAT n = 7) (one-way ANOVA with Tukey’s multiple comparison; HFD: F(2,22)=0.0063, p(sst vs agrp)=0.9996, p(sst vs vgat)=0.9935, p(agrp vs vgat)=0.9962; chow: F(2,22)=5.442, p(sst vs agrp)=0.831, p(sst vs vgat)=0.418, p(agrp vs vgat)=0.0126). All data in figure are presented as mean values±SEM.
Supplementary information
Supplementary Information
Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Mohammad, H., Senol, E., Graf, M. et al. A neural circuit for excessive feeding driven by environmental context in mice. Nat Neurosci 24, 1132–1141 (2021). https://doi.org/10.1038/s41593-021-00875-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00875-9
This article is cited by
-
Four change-makers seek impact in medical research
Nature (2024)
-
Current perspectives on brain circuits involved in food addiction-like behaviors
Journal of Neural Transmission (2024)
-
Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding
Nature Metabolism (2022)
-
Brain circuits for promoting homeostatic and non-homeostatic appetites
Experimental & Molecular Medicine (2022)
-
Two opposing hippocampus to prefrontal cortex pathways for the control of approach and avoidance behaviour
Nature Communications (2022)