Abstract
Fear and trauma generate some of the longest-lived memories. Despite the corresponding need to understand how such memories can be attenuated, the underlying brain circuits remain unknown. Here, combining viral tracing, neuronal activity mapping, fiber photometry, chemogenetic and closed-loop optogenetic manipulations in mice, we show that the extinction of remote (30-day-old) fear memories depends on thalamic nucleus reuniens (NRe) inputs to the basolateral amygdala (BLA). We found that remote, but not recent (1-day-old), fear extinction activates NRe-to-BLA inputs, which become potentiated upon fear reduction. Furthermore, both monosynaptic NRe-to-BLA and total NRe activity increase shortly before freezing cessation, suggesting that the NRe registers and transmits safety signals to the BLA. Accordingly, pan-NRe and pathway-specific NRe-to-BLA inhibition impairs, whereas their activation facilitates, remote fear extinction. These findings identify the NRe as a crucial BLA regulator for extinction and provide the first functional description of the circuits underlying the attenuation of consolidated fear memories.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are available from the authors upon reasonable request.
Code availability
All code used in this manuscript is available at https://zenodo.org/record/4588295.
References
Davidson, J. R. T., Stein, D. J., Shalev, A. Y. & Yehuda, R. Posttraumatic stress disorder: acquisition, recognition, course, and treatment. J. Neuropsychiatry Clin. Neurosci. 16, 135–147 (2014).
Foa, E. B. & Kozak, M. J. Emotional processing of fear: exposure to corrective information. Psychol. Bull. 99, 20–35 (1986).
Cukor, J., Olden, M., Lee, F. & Difede, J. Evidence-based treatments for PTSD, new directions, and special challenges. Ann. N. Y. Acad. Sci. 1208, 82–89 (2010).
Costanzi, M., Cannas, S., Saraulli, D., Rossi-Arnaud, C. & Cestari, V. Extinction after retrieval: effects on the associative and nonassociative components of remote contextual fear memory. Learn. Mem. 18, 508–518 (2011).
Gräff, J. et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 156, 261–276 (2014).
Kearns, M. C., Ressler, K. J., Zatzick, D. & Rothbaum, B. O. Early interventions for PTSD: a review. Depression Anxiety 29, 833–842 (2012).
Centonze, D., Siracusano, A., Calabresi, P. & Bernardi, G. Removing pathogenic memories: a neurobiology of psychotherapy. Mol. Neurobiol. 32, 123–132 (2005).
Marek, R., Sun, Y. & Sah, P. Neural circuits for a top-down control of fear and extinction. Psychopharmacology 236, 313–320 (2019).
Herry, C. et al. Neuronal circuits of fear extinction. Eur. J. Neurosci. 31, 599–612 (2010).
Fullana, M. A. et al. Fear extinction in the human brain: a meta-analysis of fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 88, 16–25 (2018).
Pape, H.-C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–436 (2010).
Myers, K. M. & Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 12, 120–150 (2007).
Frankland, P. W. & Bontempi, B. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 (2005).
Albo, Z. & Gräff, J. The mysteries of remote memory. Philos. Trans. R. Soc. B Biol. Sci. 373, 1742 (2018).
Knapska, E. et al. Functional anatomy of neural circuits regulating fear and extinction. Proc. Natl Acad. Sci. USA 109, 17093–17098 (2012).
Bloodgood, D. W., Sugam, J. A., Holmes, A. & Kash, T. L. Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl. Psychiatry 8, 60 (2018).
Gale, G. D. et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J. Neurosci. 24, 3810–3815 (2004).
Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017).
Silva, B. A., Burns, A. M. & Gräff, J. A cFos activation map of remote fear memory attenuation. Psychopharmacology 236, 369–381 (2018).
Khalaf, O. et al. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360, 1239–1242 (2018).
Tervo, D. G. R. et al. A designer AAV variant permits efficient retrograde access to projection. Neurons Neuron 92, 372–382 (2016).
Vetere, G. et al. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94, 363–374 (2017).
Sierra, R. O. et al. Reconsolidation-induced rescue of a remote fear memory blocked by an early cortical inhibition: involvement of the anterior cingulate cortex and the mediation by the thalamic nucleus reuniens. Hippocampus 27, 596–607 (2017).
Troyner, F., Bicca, M. A. & Bertoglio, L. J. Nucleus reuniens of the thalamus controls fear memory intensity, specificity and long-term maintenance during consolidation. Hippocampus 28, 602–616 (2018).
Schwarz, L. A. et al. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015).
Barnett, L. M., Hughes, T. E. & Drobizhev, M. Deciphering the molecular mechanism responsible for GCaMP6m’s Ca2+-dependent change in fluorescence. PLoS ONE 12, e0170934 (2017).
Vertes, R. P., Hoover, W. B., Do Valle, A. C., Sherman, A. & Rodriguez, J. J. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J. Comp. Neurol. 499, 768–796 (2006).
Kim, W. Bin & Cho, J. H. Encoding of discriminative fear memory by input-specific LTP in the amygdala. Neuron 95, 1129–1146 (2017).
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (American Psychiatric Association, 2013).
Milekic, M. H. & Alberini, C. M. Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36, 521–525 (2002).
Frankland, P. W. et al. Stability of recent and remote contextual fear memory. Learn. Mem. 13, 451–457 (2006).
An, X., Yang, P., Chen, S., Zhang, F. & Yu, D. An additional prior retrieval alters the effects of a retrieval-extinction procedure on recent and remote fear memory. Front. Behav. Neurosci. 11, 259 (2018).
Cholvin, T. et al. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J. Neurosci. 33, 8772–8783 (2013).
Xu, W. & Südhof, T. C. A neural circuit for memory specificty and generalization. Science 339, 1290–1295 (2013).
Quirk, G. J. & Mueller, D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72 (2008).
Troyner, F. & Bertoglio, L. J. Nucleus reuniens of the thalamus controls fear memory reconsolidation. Neurobiol. Learn. Mem. 177, 107343 (2021).
Ehrlich, I. et al. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771 (2009).
Likhtik, E., Popa, D., Apergis-Schoute, J., Fidacaro, G. A. & Paré, D. Amygdala intercalated neurons are required for expression of fear extinction. Nature 454, 642–645 (2008).
Salinas-Hernández, X. I. et al. Dopamine neurons drive fear extinction learning by signaling the omission of expected aversive outcomes. eLife 7, e38818 (2018).
Anderson, M. C., Bunce, J. G. & Barbas, H. Prefrontal–hippocampal pathways underlying inhibitory control over memory. Neurobiol. Learn. Mem. 134, 145–161 (2016).
Tao, Y. et al. Projections from infralimbic cortex to paraventricular thalamus mediate fear extinction retrieval. Neurosci. Bull. 37, 229–241 (2020).
Ramanathan, K. R., Jin, J., Giustino, T. F., Payne, M. R. & Maren, S. Prefrontal projections to the thalamic nucleus reuniens mediate fear extinction. Nat. Commun. 9, 4527 (2018).
Ramanathan, K. R. & Maren, S. Nucleus reuniens mediates the extinction of contextual fear conditioning. Behav. Brain Res. 374, 112114 (2019).
Silva, B. A., Gross, C. T. & Gräff, J. The neural circuits of innate fear: detection, integration, action, and memorization. Learn. Mem. 23, 544–555 (2016).
McKenna, J. T. & Vertes, R. P. Afferent projections to nucleus reuniens of the thalamus. J. Comp. Neurol. 480, 115–142 (2004).
Salay, L. D., Ishiko, N. & Huberman, A. D. A midline thalamic circuit determines reactions to visual threat. Nature 557, 183–189 (2018).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22, 460–469 (2019).
Wheeler, A. L. et al. Identification of a functional connectome for long-term fear memory in mice. PLoS Comput. Biol. 9, e1002853 (2013).
Do-Monte, F. H., Quinõnes-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015).
Paxinos, G. & Franklin, K. B. J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates (Academic Press, 2001).
Sunkin, S. M. et al. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 41, 996–1008 (2013).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
R Core Team. R: A Language and Environment for Statistical Computing. http://www.R-project.org/ (R Foundation for Statistical Computing, 2017).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Han, X. et al. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Front. Syst. Neurosci. 5, 18 (2011).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Acknowledgements
We thank M. Curdy, F. Wyler, M. Santoboni, M. Kintscher, F. Habibollahi, C. Yildirim, R. Gros, A. Goossens, C. Benquet, D. Joly, T. Hübscher, A.-R. Warter, L. Duvaud, L. Ho Dac and N. Sato for their contributions in histological procedures, the EPFL BIOP core facility for their technical assistance with image analysis, the Bertarelli Foundation Gene Therapy Core Facility at EPFL for AAV production and L. de Lecea, S. Tyree (Stanford) and N. Banterle (EPFL) for their assistance with fiber photometry. Funding: B.A.S. is supported by an EMBO long-term fellowship (ALT1605-2014, Marie Curie Actions, LTFCOFUND2013, GA-2013-609409). M.F.M.-R. is supported by an UNAM-DGECI International Scholarship from the National Autonomous University of Mexico (DGECI/DG/DFI/SME/1731/2016). J.G. is an MQ fellow (MQ15FIP100012) and a NARSAD Independent Investigator (24497). H.H. is supported by a NENS Exchange Grant. The laboratory of J.G. is supported by the European Research Council (ERC-2015-StG 678832), the Swiss National Science Foundation (31003 A_155898), the National Competence Center for Research (NCCR) SYNAPSY (51NF40-185897) and the Vallee Foundation (VS-2019-27). Work from the C.S. laboratory is supported by grants from the SNSF (31003A-176206) and the NCCR SYNAPSY (158776 and 185897).
Author information
Authors and Affiliations
Contributions
This study was planned and conceptualized by B.A.S. and J.G. B.A.S. carried out the experiments and analyzed data. S.A. performed and analyzed electrophysiological experiments under the guidance of C.S. A.M.B. contributed to fiber photometry data analysis. G.S. contributed to viral injections and behavioral experiments. H.H., L.v.d.H. and M.F.M.-R. contributed to histology and image analysis. The paper was written by B.A.S. and J.G. and commented on by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Larry Zweifel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 cFos and AAV2r quantifications in the IL, NRe and BLA upon recent and remote fear memory extinction.
a, Quantification of cFos+ cells in the IL (left) and NRe (right) of brain sections used for functional connectivity analysis following recent fear memory extinction (Fig. 1). Unpaired t-test. IL: P = 0.6748, N = 10 mice/group; NRe: P = 0.381, N = 8 (Ext), 10 (No shock) animals/group. b, Quantification of AAV2r+ cells in the IL and NRe of brain sections used for functional connectivity analysis following recent fear memory extinction (Fig. 1). Unpaired t-test. IL→BLA: P = 0.876, N = 8 (Ext), 8 (No shock) animals/group; NRe→BLA: P = 0.3599, N = 8 (Ext), 10 (No shock) animals/group; IL→NRe: P = 0.5688, N = 10 (Ext), 10 (No shock) animals/group. c, Quantification of cFos+ cells in the IL (left) and NRe (right) of brain sections used for functional connectivity analysis following remote fear memory extinction (Fig. 1). Unpaired t-test. IL: P = 0.1374, N = 9 (Ext), 8 (No shock) animals/group; NRe: P = 0.1349, N = 11 (Ext), 6 (No shock) animals/group. d, Quantification of AAV2r+ cells in the IL and NRe of brain sections used for functional connectivity analysis following remote fear memory extinction. Unpaired t-test. IL→BLA: P = 0.006, N = 9 (Ext), 8 (No shock) animals/group; NRe→BLA: P = 0.638, N = 11 (Ext), 6 (No shock) animals/group; IL→NRe: P = 0.0132, N = 9 (Ext), 9 (No shock) animals/group. e, Matched comparisons (within the same animals/group) of IL→BLA vs IL→NRe activation upon recent (left) or remote (right) fear memory extinction. Recent: Unpaired t-test: P = 0.1304, N = 7 animals/behavioral group. Two-tailed one-sample t-test (theoretical mean=1), IL→BLA, #P = 0.04; IL→NRe, P = 0.4476; Remote: Unpaired t-test: P = 0.0001, N = 9 animals/behavioral group. Two-tailed one-sample t-test (theoretical mean=1), IL→BLA, P = 0.6634; IL→NRe, ###P < 0.0001. BLA, basolateral amygdala; CFC, contextual fear conditioning; Ext, extinction; IL, infralimbic cortex; NRe, nucleus reuniens of the thalamus. Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
Extended Data Fig. 2 cFos mapping in BLA inputs.
Schematic representation of a, AAV2r injections into the BLA and b, behavioral groups used for cFos IHC in AAV2r-injected animals. Colocalization analysis of retrogradely traced cells from the BLA (AAV2r+) and cells activated upon remote fear memory recall and extinction (cFos+) in c, cortex: Two-way ANOVA, Control vs Extinction, F(1,39)=0.245, P = 0.62. Multiple comparison, Sidak. PL, N = 7 (No shock), 9 (Ext) mice/group; ACC, N = 8 (No shock), 9 (Ext) mice/group; IC, N = 6 (No shock), 6 (Ext) mice/group; d, midbrain: Two-way ANOVA, Control vs Extinction, F(1,46)=6.411, P = 0.0148. Multiple comparison, Sidak * P < 0.05. SUM, N = 6 (No shock), 19 (Ext) mice/group; VTA, N = 7 (No shock), 18 (Ext) mice/group; e, midline thalamus: Two-way ANOVA, Control vs Extinction, F(1,50)=3.989, P = 0.051. Multiple comparison, Sidak. Rh, N = 6 (No shock), 9 (Ext) mice/group; PVT, N = 8 (No shock), 7 (Ext) mice/group; IMD N = 7 (No shock), 6 (Ext) mice/group; CM, N = 8 (No shock), 7 (Ext) mice/group. f, hippocampus: unpaired t test P = 0.979. N = 7 (No shock), 8 (Ext) mice/group. ACC, anterior cingulate cortex; BLA, basolateral amygdala; CM, centromedial thalamus; IL, infralimbic cortex; IC, insular cortex (posterior, granular cortex); IMD, intermediodorsal nucleus of the thalamus; NRe, nucleus reuniens of the thalamus; PL, prelimbic cortex; PVT, periventricular thalamus; Rh, rhomboid nucleus; SUM, supramammilary nucleus; vCA1, ventral CA1; VTA, ventral tegmental area. Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
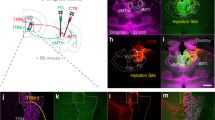
Extended Data Fig. 3 TRIO of a direct IL-NRe-BLA di-synaptic circuit.
(a) Schematic representation of the strategy for tracing the relationship between input and output (TRIO). An AAV2r-Cre virus was injected bilaterally into the BLA and Cre-dependent helper viruses (AAV8-DIO-TVA, AAV8-DIO-RG-GFP) were injected in the NRe. Retrograde transport of the AAV2r allows for selective expression of the Cre recombinase in NRe neurons projecting to the BLA. After Cre recombination, the helper AAVs express TVA, an avian receptor protein that confers infection capability to EnvA-pseudotyped rabies virus, and rabies glycoprotein B19G-GFP, which mediates the monosynaptic retrograde spread of rabies particles. Three weeks later, an EnvA-pseudotyped rabies virus carrying an mCherry coding sequence was injected into the NRe, which selectively infects TVA-expressing neurons and spreads retrogradely and trans-synaptically. (b) (Left) Example picture of starter cells in the NRe co-labeled with RG-GFP deriving from the Cre-dependent AAV and mCherry from the rabies virus. Scale bar = 250 μm. Zoom-in inset: scale bar = 100 μm. (Right) Retrogradely traced neurons in the IL identified by mCherry expression of transynaptically transported rabies. Scale bar = 250 μm. Zoom-in inset: scale bar = 100 μm. Arrowheads indicate mCherry+ neurons in the IL. (c) Schematic representation of mCherry+ neurons in the IL of one animal subjected to TRIO. (d) Quantification of the total number of mCherry+ neurons in the IL following TRIO tracing in experimental and control animals. N = 2 (Ctrl), 5 (TRIO) mice/group. (e) Schematic representation (top) and example pictures (bottom) of TRIO controls that did not receive AAV8-DIO-RG-GFP injections in the NRe. Data are represented as mean ± SEM. BLA, basolateral amygdala; IL, infralimbic cortex; NRe, nucleus reuniens of the thalamus; RG, rabies protein G.
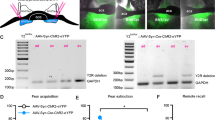
Extended Data Fig. 4 Chemogenetic inhibition of the NRe induces persistent extinction impairment and is not due to unspecific CNO effects.
(a) Freezing responses (bottom) during the remote fear memory extinction paradigm (top) upon hM4Di/CNO inhibition of the NRe. Yellow bars indicate CNO administration during extinction but not at recall or SR. Control animals were injected with a homologous virus lacking the hM4Di sequence (AAV8-hSyn::mCherry) and received CNO administration identical to experimental animals. Two-way RM ANOVA, VEH vs CNO: F(1, 19)=7.047, P = 0.0156, multiple comparison, Sidak, * P < 0.05; N = 8(hM4Di), 13 (mCherry) animals/group. (b) Freezing responses (bottom) during the remote fear memory extinction paradigm (top) upon CNO administration in hM4Di or mCherry-injected animals in the NRe. Yellow bar indicates CNO inhibition at SR only. Two-way RM ANOVA, VEH vs CNO: F(1, 12)=0.14, P = 0.72, N = 6 (CNO), 8 (VEH) mice/group. (c) Representative picture of hM4Di-mCherry expression (virus: AAV8-hM4Di-mCherry) in a mis-injected animal. Scale bar = 800 μm. Zoom-in 200 μm. (d) Experimental timeline for CNO-mediated inhibition during remote fear memory recall, extinction and SR (top). Freezing responses (bottom) during the remote fear memory extinction paradigm and SR upon hM4Di-mediated inhibition of the NRe or its vehicle control. Yellow bars indicate days of CNO administration. Two-way RM ANOVA, VEH vs CNO: F(1, 41)=0.05, P = 0.82, N = 11 (CNO), 28 (VEH) mice/group. BL, baseline freezing; CFC, contextual fear conditioning; CNO, clozapine-N-oxide; NRe, nucleus reuniens of the thalamus; Rec, recall; SR, spontaneous recovery; VEH, vehicle. Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
Extended Data Fig. 5 NRe activation during remote fear memory extinction facilitates freezing reduction.
(a) Representative pictures of hM3Dq-mCherry expression (red) and cFos (green) in the NRe upon vehicle or CNO injection. Scale bar = 200 μm. (b) Quantification of cFos-positive cell density in the NRe upon vehicle or CNO injection. Unpaired t-test, P = 0.013. N = 5 (CNO), 6 (VEH) mice/group. (c) (Top) Experimental timeline: All animals underwent contextual fear conditioning (CFC) and received AAV8-CamKII::hM3Dq-mCherry injection one week later. Thirty days after CFC they underwent memory recall and subsequently the spaced extinction paradigm under CNO or vehicle treatment and were tested for spontaneous recovery (SR) two weeks later. (Bottom) Freezing responses during the remote fear memory extinction paradigm and SR upon hM3Dq/CNO activation of the NRe. Blue bars indicate days of CNO exposure. Two-way RM ANOVA, VEH vs CNO: F(1, 10)=4.227, P = 0.0668, multiple comparison, Sidak, * p < 0.05, N = 6 mice/behavioral group. (d) Freezing responses (Bottom) during the extinction memory test (ET) and SR (top) upon repeated hM3Dq-mediated home cage activation of the NRe in the absence of both the recall and the spaced extinction behavioral paradigm. Two-way RM ANOVA, VEH vs CNO: F(1, 14)=1.18, P = 0.296, N = 8 mice/group. BL, baseline freezing; CFC, contextual fear conditioning; CNO, clozapine-N-oxide; ET, extinction test; NRe, nucleus reuniens of the thalamus; Rec, recall; SR, spontaneous recovery; VEH, vehicle. Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
Extended Data Fig. 6 Activity rise in the NRe starts shortly before freezing cessation throughout remote fear memory extinction and is not time-locked to freezing initiation.
a, (Left) Example traces of photometry signals (reported as dF/F, see Methods) generated by 465 nm (black, Ca2+-dependent) and 405 nm (blue, Ca2+-independent) LED excitation during the first three days of extinction. Blue boxes indicate freezing bouts (0.5 s ≥ light blue < 1.5 s; dark blue ≥ 1.5 s). (Right) Mean signal ±2 s around cessation of freezing (indicated by the dashed lines) for >1.5 s freezing bouts from the corresponding behavioral session in the left panel. b,c, Heat maps represent photometry signals (reported as dF/F) for each freezing epoch and the following mobility epoch of one representative recall (b), and extinction (c), session. All heat maps are aligned to freezing cessation (0 s) and ordered by freezing epoch duration. Freezing epochs followed by mobility epochs shorter that 0.1 s were excluded from the analysis.
Extended Data Fig. 7 Characterization of NRe outputs in behaviorally naïve animals.
a, (Left) Schematic representation and representative picture (scale bar = 250 μm) of AAV8-CamKII::hM3Dq-mCherry injection in the NRe. (Right) Representative picture of hM3Dq-mCherry axonal expression in the medial prefrontal cortex (mPFC; left, scale bar = 250 μm), hippocampus (top-right, scale bar = 250 μm) and amygdala (bottom-right, scale bar = 100 μm); red=mCherry, blue=Hoechst. b, Representative pictures of cFos immunoreactivity in the mPFC (left), hippocampus (top right) and amygdala (bottom right) upon chemogenetic activation of the NRe. Animals were sacrificed 90 min after systemic CNO or vehicle administration. Scale bar 200 μm. c, Quantification of cFos immunoreactivity following NRe chemogenetic activation in the mPFC, two-way ANOVA, VEH vs CNO: F(1, 18)=2.82, P = 0.11, N = 5 (CNO), 6 (VEH) mice/group; hippocampus, two-way ANOVA, VEH vs CNO: F(1, 27)=3.17, P = 0.09, N = 5 (CNO), 6 (VEH) mice/group, and the amygdala, two-way ANOVA, VEH vs CNO: F(1, 35)=22.32, P < 0.0001, multiple comparison, Sidak * P < 0.05 N = 5 (CNO), 6 (VEH) mice/group. d, Schematic representation of AAV1-hSyn::Chronos-Tom injections in the NRe and subsequent ex vivo patch-clamp recordings in neurons of the central amygdala (CeA), and pyramidal cells from the basolateral amygdala (BLA) and the lateral amygdala (LA). e, (Left) Representative traces of evoked postsynaptic currents (EPSCs) elicited at −70 mV by brief consecutive LED pulses and (right) values of EPSC peak. One-way ANOVA: F(2, 32)=17.31, P < 0.0001; multiple comparison, Holm-Sidak, *** P < 0.001, n = 10 (CeA), 8 (LA), 15 (BLA) cells from N = 3-6 mice/group. f, Paired-pulse ratio (PPR) in the different amygdalar nuclei (color-coded). One-way ANOVA F(2, 28)=7.39, P = 0.0026; multiple comparison, Holm-Sidak, * P < 0.05, ** P < 0.01, n = 9 (CeA), 7 (LA), 15 (BLA) cells from N = 3-6 mice/group. BLA, basolateral amygdala, CeAl, lateral portion of the central amygdala, CeAm, medial portion of the central amygdala; DG, dentate gyrus; IL, infralimbic cortex; LA, lateral amygdala; mPFC, medial prefrontal cortex; PL, prelimbic cortex. Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
Extended Data Fig. 8 cFos activation analysis of NRe→BLA projections during remote fear memory extinction.
a, Representative pictures of cFos IHC in retrogradely traced cells from the BLA in the NRe following the remote recall session, the last extinction session and the no shock control group (for the experimental setup see Fig. 1). Scale bar = 100 μm. Arrowheads indicate double positive cells in the NRe (AAV2r+, cFos+). b, Colocalization analysis of retrogradely traced cells from the BLA (AAV2r+) and cells activated in the NRe (cFos+) in the remote recall, extinction and the no shock control group. Kruskal–Wallis test, P = 0.02, multiple comparison, Dunn * P < 0.05, ** P < 0.01, N = 6 (No shock), 14 (Recall), 11 (Ext) mice/group; Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
Extended Data Fig. 9 cFos activation and functional analysis of IL→BLA projections during remote fear memory extinction.
a, Schematic representation of the retrograde virus injection strategy. b, Representative pictures of cFos IHC in retrogradely traced cells from the BLA in the IL following the remote recall session, the last extinction session and the no shock control group (for experimental setup see Fig. 1). Scale bar = 100 μm c, Colocalization analysis of retrogradely traced cells from the BLA (AAV2r+) and cells activated in the IL (cFos+) in the remote recall, remote extinction and the no shock control group. Kruskal-Wallis test, multiple comparison, Dunn; P = 0.89, N = 8 (No shock), 7 (recall), 9 (Ext) mice/group. d, (Left) Schematic representation of the experimental strategy for DREADD-mediated manipulation of IL-deriving terminals in the BLA. (Right) Representative picture of hM4Di-mCherry expression at the level of the injection site in the IL (top) and cannula placement in the BLA (bottom). Notably, hM4Di-mCherry+ fibers can be observed in the NRe and BLA (scale bar = 500 μm). e, Selective hM4Di-mediated inhibition of IL-deriving terminals in the BLA does not affect remote fear memory extinction. Yellow bars indicate local BLA infusions of CNO/VEH at remote recall and throughout the remote fear memory extinction paradigm. Two-way RM ANOVA, VEH vs CNO: F(1, 7)=0.234, P = 0.643, multiple comparison, Sidak, N = 4 (VEH), 5 (CNO) mice/group. Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
Extended Data Fig. 10 Functional characterization of IL→NRe projections during remote fear memory extinction.
a, Schematic representation of the retrograde virus injection strategy. b, Representative pictures of cFos IHC in retrogradely traced cells from the NRe in the IL following the remote recall session, the last extinction session and the no shock control group (for experimental setup see Fig. 1). c, Colocalization analysis of retrogradely traced cells from the NRe (AAV2r+) and cells activated in the IL (cFos+) in the remote recall, remote extinction and the no shock control group. Kruskal-Wallis test, P = 0.031, N = 7 (No shock), 7 (recall), 9 (Ext) mice/group; multiple comparison, Dunn, * P < 0.05. Arrowheads indicate double positive cells in the IL (AAV2r+, cFos+). d, Schematic representation (left) and representative pictures (right) of the viral strategy for optogenetic stimulation of IL→NRe projections. AAV8 vectors carrying the activatory opsin Chronos-mCherry or mCherry only were injected bilaterally in the IL and an optic fiber was implanted above the NRe. Scale bar= 500 μm. e, Freezing behavior quantification upon optogenetic stimulation (20 Hz, 5 s light ON, 5 s light OFF, total duration=3 min) of IL→NRe projections during one remote fear memory extinction session. Two-way ANOVA for freezing time during light ON and OFF periods during one remote extinction session, interaction: F(1, 7)=17.42, P = 0.0042; ON vs OFF: F(1, 7)=21.49, P = 0.0024, multiple comparison, Sidak, * P < 0.05, ** P < 0.01, *** P < 0.001. N = 4 (Chronos), 5 (mCherry) mice/group. f, Schematic representation of the experimental approach and fiber photometry recording implant for IL→NRe fiber photometry recordings (top); representative picture of GCaMP6f expression and localization of the optical fiber implant in the IL (bottom). Scale bar = 500 μm. g, (Left) Example traces of photometry signals (reported as dF/F, see Methods) generated by 465 nm (black, Ca2+-dependent) and 405 nm (blue, Ca2+-independent) LED excitation during habituation, recall and the last extinction session. Blue boxes indicate freezing bouts (0.5 s ≥ light blue < 1.5 s; dark blue ≥1.5 s). (Right) Mean dF/F signal ±2 s around cessation of freezing (indicated by the dashed line, 0 s) for ≥1.5 s freezing bouts from the corresponding behavioral session in the left panel. h, (Left) Quantification of dF/F difference before and after freezing end. RM One-way ANOVA, F(2, 8)=0.989, P = 0.41, multiple comparison, Holm-Sidak, N = 5 animals. (Right) Signal power analysis of dF/F during remote fear memory extinction. Signal power was calculated as Σ(dF/F–av.dF/F)2. RM One-way ANOVA, F(2, 8)=6.72, P = 0.019, multiple comparison, Sidak, * P < 0.05. N = 5 animals. Data are represented as mean ± SEM. Statistical analysis details for each figure panel are reported in Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Silva, B.A., Astori, S., Burns, A.M. et al. A thalamo-amygdalar circuit underlying the extinction of remote fear memories. Nat Neurosci 24, 964–974 (2021). https://doi.org/10.1038/s41593-021-00856-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00856-y
This article is cited by
-
A prefrontal-thalamic circuit encodes social information for social recognition
Nature Communications (2024)
-
Context Processing in Contextual and Cued Fear Extinction
Neuroscience Bulletin (2024)
-
Xiphoid nucleus of the midline thalamus controls cold-induced food seeking
Nature (2023)
-
Dopamine D1-like receptors modulate synchronized oscillations in the hippocampal–prefrontal–amygdala circuit in contextual fear
Scientific Reports (2023)
-
A thalamic-hippocampal CA1 signal for contextual fear memory suppression, extinction, and discrimination
Nature Communications (2023)