Abstract
Social interactions and relationships are often rewarding, but the neural mechanisms through which social interaction drives positive experience remain poorly understood. In this study, we developed an automated operant conditioning system to measure social reward in mice and found that adult mice of both sexes display robust reinforcement of social interaction. Through cell-type-specific manipulations, we identified a crucial role for GABAergic neurons in the medial amygdala (MeA) in promoting the positive reinforcement of social interaction. Moreover, MeA GABAergic neurons mediate social reinforcement behavior through their projections to the medial preoptic area (MPOA) and promote dopamine release in the nucleus accumbens. Finally, activation of this MeA-to-MPOA circuit can robustly overcome avoidance behavior. Together, these findings establish the MeA as a key node for regulating social reward in both sexes, providing new insights into the regulation of social reward beyond the classic mesolimbic reward system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated and analyzed during this study are either included in this published article or available from the corresponding author upon reasonable request.
Code availability
Behavior was analyzed using MATLAB code, available at https://pdollar.github.io/toolbox/. The code that supports these findings is available upon reasonable request from the corresponding author.
References
Stanley, D. A. & Adolphs, R. Toward a neural basis for social behavior. Neuron 80, 816–826 (2013).
Umberson, D., Crosnoe, R. & Reczek, C. Social relationships and health behavior across the life course. Annu. Rev. Socio. 36, 139–157 (2010).
Chen, P. & Hong, W. Neural circuit mechanisms of social behavior. Neuron 98, 16–30 (2018).
Tamir, D. I. & Hughes, B. L. Social rewards: from basic social building blocks to complex social behavior. Perspect. Psychol. Sci. 13, 700–717 (2018).
Hu, H. Reward and aversion. Annu. Rev. Neurosci. 39, 297–324 (2016).
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S. & Schultz, R. T. The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239 (2012).
Zeeland, A. A. S.-V., Dapretto, M., Ghahremani, D. G., Poldrack, R. A. & Bookheimer, S. Y. Reward processing in autism. Autism Res. 3, 53–67 (2010).
Nieh, E. H. et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron 90, 1286–1298 (2016).
Hung, L. W. et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017).
Dölen, G., Darvishzadeh, A., Huang, K. W. & Malenka, R. C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179 (2013).
McHenry, J. A. et al. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci. 20, 449–458 (2017).
Hong, W., Kim, D.-W. & Anderson, D. J. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158, 1348–1361 (2014).
Chen, P. B. et al. Sexually dimorphic control of parenting behavior by the medial amygdala. Cell 176, 1206–1221 (2019).
Lin, D. et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011).
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M. & Dulac, C. G. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325 (2014).
Falkner, A. L., Grosenick, L., Davidson, T. J., Deisseroth, K. & Lin, D. Hypothalamic control of male aggression-seeking behavior. Nat. Neurosci. 19, 596–604 (2016).
Miller, S. M., Marcotulli, D., Shen, A. & Zweifel, L. S. Divergent medial amygdala projections regulate approach-avoidance conflict behavior. Nat. Neurosci. 22, 565–575 (2019).
Gutzeit, V. A. et al. Optogenetic reactivation of prefrontal social neural ensembles mimics social buffering of fear. Neuropsychopharmacology 45, 1068–1077 (2020).
Swanson, L. W. Cerebral hemisphere regulation of motivated behavior. Brain Res. 886, 113–164 (2000).
Choi, G. B. et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46, 647–660 (2005).
Li, Y. et al. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171, 1176–1190 (2017).
Bergan, J. F., Ben-Shaul, Y. & Dulac, C. Sex-specific processing of social cues in the medial amygdala. eLife 3, e02743 (2014).
Ishii, K. K. et al. A labeled-line neural circuit for pheromone-mediated sexual behaviors in mice. Neuron 95, 123–137 (2017).
Unger, E. K. et al. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10, 453–462 (2015).
Yao, S., Bergan, J., Lanjuin, A. & Dulac, C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. eLife 6, e31373 (2017).
Demir, E. et al. The pheromone darcin drives a circuit for innate and reinforced behaviours. Nature 578, 137–141 (2020).
Golden, S. A. et al. Compulsive addiction-like aggressive behavior in mice. Biol. Psychiatry 82, 239–248 (2017).
Venniro, M. et al. Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 21, 1520–1529 (2018).
Martin, L. & Iceberg, E. Quantifying social motivation in mice using operant conditioning. J. Vis. Exp. e53009 (2015).
Jacobs, S., Huang, F., Tsien, J. & Wei, W. Social recognition memory test in rodents. Bio-protocol 6, e1804 (2016).
Hlinák, Z. & Krejcí, I. Social recognition in male rats: age differences and modulation by MIF-I and Alaptide. Physiol. Res. 40, 59–67 (1991).
Berke, J. D. What does dopamine mean? Nat. Neurosci. 21, 787–793 (2018).
Volkow, N. D., Wise, R. A. & Baler, R. The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci. 18, 741–752 (2017).
Fang, Y.-Y., Yamaguchi, T., Song, S. C., Tritsch, N. X. & Lin, D. A hypothalamic midbrain pathway essential for driving maternal behaviors. Neuron 98, 192–207.e10 (2018).
Kohl, J. et al. Functional circuit architecture underlying parental behaviour. Nature 556, 326–331 (2018).
Hennessy, M. B., Kaiser, S. & Sachser, N. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30, 470–482 (2009).
Nardou, R. et al. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 569, 116–120 (2019).
Schultz, W. Dopamine reward prediction-error signalling: a two-component response. Nat. Rev. Neurosci. 17, 183–195 (2016).
Collins, A. L. et al. Dynamic mesolimbic dopamine signaling during action sequence learning and expectation violation. Sci. Rep. 6, 20231 (2016).
Prévost-Solié, C., Girard, B., Righetti, B., Tapparel, M. & Bellone, C. Dopamine neurons of the VTA encode active conspecific interaction and promote social learning through social reward prediction error. Prerint at bioRxiv https://doi.org/10.1101/2020.05.27.118851 (2020).
Padilla, S. L. et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016).
Berke, J. D. & Hyman, S. E. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515–532 (2000).
Golden, S. A. et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534, 688 (2016).
Beny-Shefer, Y. et al. Nucleus accumbens dopamine signaling regulates sexual preference for females in male mice. Cell Rep. 21, 3079–3088 (2017).
Kingsbury, L. et al. Cortical representations of conspecific sex shape social behavior. Neuron 107, 941–953 (2020).
Wu, Y. E., Pan, L., Zuo, Y., Li, X. & Hong, W. Detecting activated cell populations using single-cell RNA-seq. Neuron 96, 313–329 (2017).
Kupferberg, A., Bicks, L. & Hasler, G. Social functioning in major depressive disorder. Neurosci. Biobehav. Rev. 69, 313–332 (2016).
Matthews, G. A. et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617–631 (2016).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Yang, C. F. et al. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153, 896–909 (2013).
Mahn, M. et al. High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins. Nat. Commun. 9, 4125 (2018).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Broussard, G. J. et al. In vivo measurement of afferent activity with axon-specific calcium imaging. Nat. Neurosci. 21, 1272–1280 (2018).
Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 (2017).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Dimidschstein, J. et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat. Neurosci. 19, 1743–1749 (2016).
Acknowledgements
We thank K. Wassum, P. Golshani, L. Kingsbury, T. Raam, D. Wei, L. Gu, F. Sun and M. Zhang for critical comments on this manuscript and P. Chen, S. Hu, H. Li, Y. Wu and P. Zhao for technical assistance. This work was supported, in part, by NIH R01 NS113124, a Searle Scholars Award, a Klingenstein-Simons fellowship, a Brain Research Foundation grant, a Packard Foundation fellowship, a McKnight Scholar Award, a Keck Foundation Award, a Vallee Scholars Award and a Mallinckrodt Scholar Award to W.H. and a Marion Bowen postdoctoral grant to R.K.H.
Author information
Authors and Affiliations
Contributions
R.K.H. and W.H. conceptualized and designed the study. R.K.H., Y.Z., T.L. and J.W. performed optogenetics and behavioral experiments. R.K.H. and T.L. performed fiber photometry and histology. R.K.H. analyzed behavioral and neural data. R.K.H. performed ex vivo electrophysiology experiments. P.M. provided suggestions on electrophysiology. R.K.H., Y.E.W. and W.H. wrote the manuscript. W.H. supervised the entire study, provided resources and acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Peer review information Nature Neuroscience thanks Sam Golden, Olga Penagarikano and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
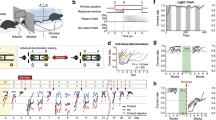
Extended Data Fig. 1 Characterizations of behaviors in free social interaction and social operant task.
a-c, Fraction of time spent on seven distinct social behaviors displayed by adult animals (a, all animals; b, males; c, females) towards juvenile animals during free social interaction. Both male and female adult animals predominantly display close social investigation (sniffing) towards juvenile animals. The behavioral characterizations of these male and female animals in the social operant task are included in Extended Data Fig. 2. d, e, Cumulative distribution of the numbers of nose pokes in social and null ports in social operant task in one representative animal on each day. The assay was performed in the presence (d) or absence (e) of the target animal. f, The majority of social port pokes are followed by close social interaction between subject and target animals (across the barrier). P = 0.2149, Paired t test (two-sided). g, Percentage of animals showing consistent preference for the social port (defined as showing >20% preference on both days 6 and 7) in the presence or absence of the target animal. h, Full distribution of nose poke preferences in individual animals in Fig. 1i. Grey lines and dots indicate preferences of individual mice and orange line indicates the average across all animals. In (a-c), n = 12 males and 8 females; in (f), n = 17 mice; (g), n = 17 mice (with target animals) and 25 mice (without target animals); in (h), n = 17 mice. (a-c, f), boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile. For detailed statistics information, see Supplementary Table 1.
Extended Data Fig. 2 Both male and female adult mice exhibit social reward behavior.
a, b, Preference for social port and numbers of pokes in females (a) and males (b) in the presence of a target animal. Both male and female animals develop strong preference for the social port in the presence of target animals. Experiment was performed over a 10-day period, which consists of 1 day of baseline session, 7 days of training sessions with target animals, and 2 days of post-training sessions. c, d, Preference for social port and numbers of pokes in females (c) and males (d) in the absence of a target animal. Neither male nor female animals develop preference for the social port. Experiment was performed over an 8-day period, which consists of 1 day of baseline session and 7 days of training sessions without target animals. e, Percentage of male and female mice exhibiting consistent preference for social port (defined as showing >20% preference on both days 6 and 7). In (a, b, e), with target animals, n = 17 females and 20 males; these include 9 females and 8 males presented in Fig. 1 as well as an additional 8 females and 12 males presented in Extended Data Fig. 1. In (c, d, e), without target animals, n = 15 females and 10 males. In (a-d), left panels, one-way repeated measures ANOVA with Bonferroni post-hoc correction (*P < 0.05, **P < 0.01, ***P < 0.001); right panels, two-way repeated measures ANOVA with Bonferroni post-hoc correction (**P < 0.01, ***P < 0.001). (a-d), mean ± SEM. For detailed statistics information, see Supplementary Table 1.
Extended Data Fig. 3 Additional characterizations of genetic ablation and optogenetic inhibition experiments.
a, b, Full distribution of nose poke preferences in individual animals in Fig. 2e (a) and Fig. 2g (b). Grey lines and dots indicate preferences of individual mice and colored line indicates the average across all animals. c, Representative trace showing robust silencing of Vgat+ neurons during light illumination. d, Firing rates before, during, and after light illumination. One-way repeated measures ANOVA with Bonferroni post-hoc correction (***P < 0.001). e, g, Fraction of time spent on seven distinct social behaviors displayed by subject animals (e, GtACR-expressing animals, or g, control animals) towards juvenile animals. Two-way repeated measures ANOVA with Bonferroni post-hoc (**P < 0.01, ***P < 0.001). f, h, Percentage of total time spent on all social behaviors displayed by subject animals (f, GtACR-expressing animals or h, control animals). **P = 0.0046 (f), P = 0.1766 (h), paired t test (two-sided). (a, b) n = 8 control mice and 10 Caspase mice; (c, d) n = 10 trials from 3 cells (2 mice); (e-h), n = 6 control mice and 5 GtACR-expressing mice. (d-h) boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile. For detailed statistics information, see Supplementary Table 1.
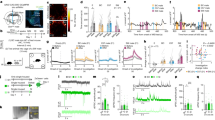
Extended Data Fig. 4 Fiber photometry of calcium signals in response to social and nonsocial rewarding stimuli.
MeApd Vgat+ neurons and their projections to the MPOA are active in response to social stimuli, but not to nonsocial rewarding stimuli, such as sucrose and chocolate. a, c, Dynamics of Ca2+ signals (ΔF/F) in MeApd Vgat+ neurons (a) or in the axonal projections of MeApd Vgat+ neurons to the MPOA (c) in response to social stimuli (juveniles) or during consumption of sucrose solution or chocolate. b, d, AUC per second under different conditions in a and c. Controls were done by measuring Ca2+ signals from EYFP-expressing MeApd Vgat+ neurons or EYFP-expressing MeApd Vgat+ neuron projections to the MPOA during exposure to social stimuli. One-way ANOVA with Bonferroni post-hoc correction (*P < 0.05, **P < 0.01, ***P < 0.001). (a, b), n = 15 trials (social), 20 trials (sucrose), and 11 trials (chocolate) from 6 GCaMP7f-expressing mice; n = 22 trials (social) from 4 EYFP-expressing control mice. (c, d), n = 31 trials (social), 48 trials (sucrose), and 37 trials (chocolate) from 7 GCaMP6s-expressing mice; n = 17 trials (social) from 4 EYFP-expressing control mice. (a, c), mean ± SEM; (b, d), boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile. For detailed statistics information, see Supplementary Table 1.
Extended Data Fig. 5 The MeApd-to-MPOA circuit does not mediate food reward.
a-d, Ablation of MeApd Vgat+ neurons does not affect food reward in an operant task. Animals are trained to lever press to obtain food pellets (see Methods). Control: animals expressing mCherry in MeApd Vgat+ neurons, Caspase 3: animals expressing Caspase 3 in MeApd Vgat+ neurons. e-h, Ablation of MPOA-projecting MeApd neurons does not affect food reward in an operant task. Control: animals expressing mCherry in MPOA-projecting MeApd neurons, Caspase 3: animals expressing Caspase 3 in MPOA-projecting MeApd neurons. a, b, e, f, cumulative distribution of lever presses in the operant task for food reward. c, g, number of lever presses. Two-way repeated measures ANOVA with Bonferroni post-hoc correction (***P < 0.001). d, h, preferences for the lever associated with food reward. Mann-Whitney test (two-sided). (a-d), n = 7 control mice and 6 Caspase mice; (e-h), n = 5 control mice and 6 Caspase mice; (a-h), mean ± SEM. For detailed statistics information, see Supplementary Table 1.
Extended Data Fig. 6 Activation of MeApd Vgat+ neurons promotes reinforcement behavior in both males and females.
a, Activation of MeApd Vgat+ neurons in the intracranial self-stimulation assay. ChR2-expressing animals spend greater time in the stimulation-coupled active port (total time spent in the port), whereas control animals do not. Two-way repeated measures ANOVA with Bonferroni post-hoc correction (***P < 0.001). b, e, In a real-time place preference assay, ChR2-expressing animals display a positive preference towards the stimulation-coupled chamber compared to the EYFP-expressing controls in both females (b) and males (e). *P = 0.0242 (b), *P = 0.0121 (e), Mann-Whitney test (two-sided). c, f, In an intracranial self-stimulation assay, ChR2-expressing female (c) and male (f) animals exhibit a greater number of pokes towards the active port compared to the inactive port, whereas control animals do not. Two-way repeated measures ANOVA with Bonferroni post-hoc correction (**p < 0.01, ***p < 0.001). d, g, ChR2-expressing female (d) and male (g) animals spend greater time in the active port (total time spent in the port), whereas control animals do not. Two-way repeated measures ANOVA with Bonferroni post-hoc correction (*P < 0.05, **P < 0.01, ***P < 0.001). (a), n = 6 control mice and 10 ChR2 mice; (b), n = 3 control mice and 8 ChR2 mice; (c-d), n = 3 control mice and 7 ChR2 mice; (e), n = 3 control mice and 8 ChR2 mice; (f-g), n = 3 control mice and 3 ChR2 mice. (a) boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile; (b-g) mean ± SEM. For detailed statistics information, see Supplementary Table 1.
Extended Data Fig. 7 Optogenetic activation of MeApd Vgat+ neurons or MeApd-to-MPOA projections drives reinforcement.
a-d, Cumulative distribution of the numbers of nose pokes to the optogenetic ‘social’ port or the null port in a modified social operant task. Representative data from a control or ChR2 animal (a, b, MeApd cell bodies; c, d, MeApd-to-MPOA projections) on each day. e-h, Numbers of pokes to the optogenetic ‘social’ port or the null port on each day. For MeApd cell bodies, control (e) and ChR2-expressing (f) animals; for MeApd-to-MPOA projections, control (g) and ChR2-expressing (h) animals. Two-way repeated measures ANOVA with Bonferroni post-hoc correction (**P < 0.01, ***P < 0.001). i, Activation of MeApd-to-MPOA projections in ChR2-expressing animals spend greater time in the active port (total time spent in the port), whereas control animals do not. Two-way repeated measures ANOVA with Bonferroni post-hoc correction ((*P < 0.05, **P < 0.01). (e, f), n = 6 mice (control) and n = 8 mice (ChR2); (g, h), n = 8 mice (control) and n = 8 mice (ChR2); (i), n = 5 mice (control) and 7 mice (ChR2). (e-h), mean ± SEM; (i), boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile. For detailed statistics information, see Supplementary Table 1.
Extended Data Fig. 8 Optogenetic activation of MeApd Vglut2+ neurons promotes place aversion.
a, Schematic showing the real-time place preference assay (RTPP). Light blue area (top) indicates the chamber paired with light stimulation when the mouse enters. Representative heatmaps showing locomotion trajectories for controls (middle) and ChR2-experessing (bottom) Vglut2-Cre mice in the RTPP test. b, Example image of viral expression in the MeApd of Vglut2-Cre animals. Scale bar = 200 μm. c, ChR2-expressing Vglut2-Cre animals display a negative preference towards the stimulation-couple chamber compared to EYFP-expressing controls. n = 4 mice (control) and 6 mice (ChR2). **P = 0.0095, Mann-Whitney test (two-sided). Boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile. For detailed statistics information, see Supplementary Table 1.
Extended Data Fig. 9 Behavioral functions of MeA-to-MPOA and MeA-to-PMV pathways.
a, Schematic showing optogenetic stimulation of MeApd axon terminals in the MPOA and lidocaine infusion in the MeApd cell bodies. b, Local infusion of lidocaine in the MeApd does not affect the behavioral effect of stimulating the MeApd-to-MPOA projection. P = 0.7612, paired t test (two-sided). c-d, Activating the MeApd-to-PMV projection does not promote positive reinforcement. c, Example images showing expression of ChR2 in the MeApd Vgat+ neurons (left) and fiber placement above their axon terminals in the PMV (right). Scale bar = 200 µm. d, Optogenetic stimulation of MeApd Vgat+ neuron terminals in the PMV does not produce positive place preference in a real-time place preference assay. P = 0.5103, paired t test (two-sided). e-g. Suppressing MeApd Vgat+ neuron activity reduces social preference in a three-chamber assay. e, Schematic showing the three-chamber assay. f-g, Optogenetic inhibition of MPOA-projecting MeA neurons in GtACR-expressing mice (g), but not in control mice (f), reduces social preference in a three-chamber social preference assay. P = 0.7183 (f), *P = 0.0471 (g), paired t test (two-sided). (b), n = 5 ChR2-expressing mice; (c, d), n = 6 ChR2-expressing mice; (f-g), n = 6 control mice and 5 GtACR-expressing mice. Boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile. For detailed statistics information, see Supplementary Table 1.
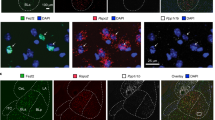
Extended Data Fig. 10 Characterization of MeApd neurons projecting to the MPOA.
a, Schematic showing injections of CTB-Alexa 647 into the MPOA and AAV-Dlx-NLS-Ruby2 and AAV-DIO-EYFP into the MeApd of Vglut2-Cre animals. b, Representative images showing labeling of CTB-Alexa 647 in the MPOA. Scale bar = 100 µm. c, Representative images showing retrogradely labeled CTB-Alexa 647 with the expression of AAV-Dlx-NLS-Ruby2 and AAV-DIO-EYFP in the MeApd of Vglut2-Cre animals. Scale bar = 20 µm. d, The fraction of overlap between Dlx+ and Vglut2+ neurons among all Dlx+ neurons. We confirm that the expression of Dlx and Vglut2 promotors shows little overlap in the MeApd. e, Fraction of MPOA-projecting MeApd neurons that are GABAergic. The majority of the MeApd neurons that project to the MPOA are GABAergic. f, Representative images showing expression of ChR2 in the MeApd Vglut2+ neurons (left) and fiber placement above their axon terminals in the MPOA (right) of Vglut2-Cre animals. Scale bar = 100 µm. g, Activating the MeApd Vglut2+ projections to the MPOA does not drive place preference. P = 0.1958, Paired t test (two-sided). (b-e), n = 3 mice; (f, g), n = 6 ChR2 mice. (d, e), mean ± SEM; (g), Boxplots: center = median, box = quartiles, whisker = 10 − 90 percentile. For detailed statistics information, see Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Table 1
Rights and permissions
About this article
Cite this article
Hu, R.K., Zuo, Y., Ly, T. et al. An amygdala-to-hypothalamus circuit for social reward. Nat Neurosci 24, 831–842 (2021). https://doi.org/10.1038/s41593-021-00828-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00828-2
This article is cited by
-
Differential contribution of canonical and noncanonical NLGN3 pathways to early social development and memory performance
Molecular Brain (2024)
-
Advancing preclinical chronic stress models to promote therapeutic discovery for human stress disorders
Neuropsychopharmacology (2024)
-
The cognitive impact of light: illuminating ipRGC circuit mechanisms
Nature Reviews Neuroscience (2024)
-
Operant social self-administration in male CD1 mice
Psychopharmacology (2024)
-
The genetic architecture of the human hypothalamus and its involvement in neuropsychiatric behaviours and disorders
Nature Human Behaviour (2024)