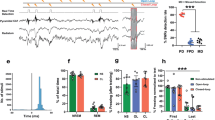
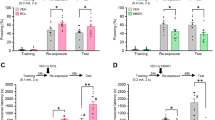
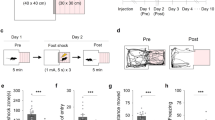
Abstract
Reconsolidation may be a viable therapeutic target to inhibit pathological fear memories. In the clinic, incidental or imaginal reminders are used for safe retrieval of traumatic memories of experiences that occurred elsewhere. However, it is unknown whether indirectly retrieved traumatic memories are sensitive to disruption. Here we used a backward (BW) conditioning procedure to indirectly retrieve and manipulate a hippocampus (HPC)-dependent contextual fear engram in male rats. We show that conditioned freezing to a BW conditioned stimulus (CS) is mediated by fear to the conditioning context, activates HPC ensembles that can be covertly captured and chemogenetically activated to drive fear, and is impaired by post-retrieval protein synthesis inhibition. These results reveal that indirectly retrieved contextual fear memories reactivate HPC ensembles and undergo protein synthesis-dependent reconsolidation. Clinical interventions that rely on indirect retrieval of traumatic memories, such as imaginal exposure, may open a window for editing or erasure of neural representations that drive pathological fear.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
References
Craske, M. G., Treanor, M., Conway, C. C., Zbozinek, T. & Vervliet, B. Maximizing exposure therapy: an inhibitory learning approach. Behav. Res. Ther. 58, 10–23 (2014).
McNally, R. J. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin. Psychol. Rev. 27, 750–759 (2007).
Vervliet, B., Craske, M. G. & Hermans, D. Fear extinction and relapse: state of the art. Annu. Rev. Clin. Psychol. 9, 215–248 (2013).
Nader, K., Schafe, G. E. & Le Doux, J. E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000).
Przybyslawski, J. & Sara, S. J. Reconsolidation of memory after its reactivation. Behav. Brain Res. 84, 241–246 (1997).
Duvarci, S. & Nader, K. Characterization of fear memory reconsolidation. J. Neurosci. 24, 9269–9275 (2004).
Kindt, M., Soeter, M. & Vervliet, B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat. Neurosci. 12, 256–258 (2009).
Phelps, E. A. & Hofmann, S. G. Memory editing from science fiction to clinical practice. Nature 572, 43–50 (2019).
Blundell, J., Kouser, M. & Powell, C. M. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol. Learn. Mem. 90, 28–35 (2008).
Debiec, J., LeDoux, J. E. & Nader, K. Cellular and systems reconsolidation in the hippocampus. Neuron 36, 527–538 (2002).
Eshuis, L. V. et al. Efficacy of immersive PTSD treatments: a systematic review of virtual and augmented reality exposure therapy and a meta-analysis of virtual reality exposure therapy. J. Psychiatr. Res. https://doi.org/10.1016/j.jpsychires.2020.11.030 (2020).
Soeter, M. & Kindt, M. Retrieval cues that trigger reconsolidation of associative fear memory are not necessarily an exact replica of the original learning experience. Front. Behav. Neurosci. 9, 122 (2015).
Runyan, J. D. & Dash, P. K. Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning. Hippocampus 15, 333–339 (2005).
Goode, T. D., Ressler, R. L., Acca, G. M., Miles, O. W. & Maren, S. Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. eLife 8, e46525 (2019).
Ressler, R. L., Goode, T. D., Evemy, C. & Maren, S. NMDA receptors in the CeA and BNST differentially regulate fear conditioning to predictable and unpredictable threats. Neurobiol. Learn. Mem. 174, 107281 (2020).
Chang, R. C., Blaisdell, A. P. & Miller, R. R. Backward conditioning: mediation by the context. J. Exp. Psychol. Anim. Behav. Process. 29, 171–183 (2003).
Maren, S., Phan, K. L. & Liberzon, I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428 (2013).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Tanaka, K. Z. et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84, 347–354 (2014).
Denny, C. A. et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014).
Chen, B. K. et al. Artificially enhancing and suppressing hippocampus-mediated memories. Curr. Biol. 29, 1885–1894 (2019).
Josselyn, S. A. & Tonegawa, S. Memory engrams: recalling the past and imagining the future. Science 367, eaaw4325 (2020).
Goode, T. D., Tanaka, K. Z., Sahay, A. & McHugh, T. J. An integrated index: engrams, place cells, and hippocampal memory. Neuron 107, 805–820 (2020).
Tonegawa, S., Morrissey, M. D. & Kitamura, T. The role of engram cells in the systems consolidation of memory. Nat. Rev. Neurosci. 19, 485–498 (2018).
Davis, P. & Reijmers, L. G. The dynamic nature of fear engrams in the basolateral amygdala. Brain Res. Bull. 141, 44–49 (2018).
Alfei, J. M. et al. Generalization and recovery of post-retrieval amnesia. J. Exp. Psychol. Gen. 149, 2063–2083 (2020).
Debiec, J., Doyère, V., Nader, K. & Ledoux, J. E. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl Acad. Sci. USA 103, 3428–3433 (2006).
Lattal, K. M. & Abel, T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc. Natl Acad. Sci. USA 101, 4667–4672 (2004).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015).
Roy, D. S., Muralidhar, S., Smith, L. M. & Tonegawa, S. Silent memory engrams as the basis for retrograde amnesia. Proc. Natl Acad. Sci. USA 114, E9972–E9979 (2017).
Trent, S., Barnes, P., Hall, J. & Thomas, K. L. Rescue of long-term memory after reconsolidation blockade. Nat. Commun. 6, 7897 (2015).
Gisquet-Verrier, P. et al. Integration of new information with active memory accounts for retrograde amnesia: a challenge to the consolidation/reconsolidation hypothesis? J. Neurosci. 35, 11623–11633 (2015).
Schroyens, N., Sigwald, E. L., Van Den Noortgate, W., Beckers, T. & Luyten, L. Reactivation-dependent amnesia for contextual fear memories: evidence for publication bias. eNeuro 8, ENEURO.0108-20.2020 (2020).
Luyten, L., Schnell, A. E., Schroyens, N. & Beckers, T. Lack of drug-induced post-retrieval amnesia for auditory fear memories in rats. BMC Biol. 19, 17 (2021).
Swanson, L. W. Brain maps 4.0-structure of the rat brain: an open access atlas with global nervous system nomenclature ontology and flatmaps. J. Comp. Neurol. 526, 935–943 (2018).
Gafford, G. M., Parsons, R. G. & Helmstetter, F. J. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience 182, 98–104 (2011).
Maren, S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J. Neurosci. 18, 3088–3097 (1998).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Marek, R. et al. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat. Neurosci. 21, 384–392 (2018).
Shrestha, P. et al. Cell-type-specific drug-inducible protein synthesis inhibition demonstrates that memory consolidation requires rapid neuronal translation. Nat. Neurosci. 23, 281–292 (2020).
Acknowledgements
We thank S. Tonegawa for kindly providing plasmids (pAAV.TRE.hM3Dq.mCherry and pAAV.cFos.tTA). We also thank J. Liu and A. Martinez for technical assistance, and M. Kindt, A. Milton, and S. Ramirez for their helpful reviews of the manuscript. This work was supported by NIH grant nos. F31MH107113 (T.D.G.), R01MH065961 and R01MH117852 (S.M.), and by a Brain & Behavioral Research Foundation Distinguished Investigator grant (S.M.).
Author information
Authors and Affiliations
Contributions
R.L.R., T.D.G. and S.M. designed the experiments, analyzed data and wrote the manuscript. R.L.R. and T.D.G. collected data for all experiments. S.K. assisted with c-Fos data collection and with the behavioral experiments shown in Fig. 1. K.R.R. assisted with the behavioral experiments shown in Fig. 3.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Merel Kindt, Amy Milton and Steve Ramirez for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Source Data Fig. 1
This file contains raw freezing values used to generate plots in Fig. 1.
Source Data Fig. 2
This file contains raw freezing values and cell counts used to generate plots in Fig. 2.
Source Data Fig. 3
This file contains raw freezing values and cell counts used to generate plots in Fig. 3.
Source Data Fig. 4
This file contains raw freezing values used to generate plots in Fig. 4.
Rights and permissions
About this article
Cite this article
Ressler, R.L., Goode, T.D., Kim, S. et al. Covert capture and attenuation of a hippocampus-dependent fear memory. Nat Neurosci 24, 677–684 (2021). https://doi.org/10.1038/s41593-021-00825-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00825-5
This article is cited by
-
Rapamycin attenuates reconsolidation of a backwards-conditioned aversive stimuli in female mice
Psychopharmacology (2024)
-
An automated, low-latency environment for studying the neural basis of behavior in freely moving rats
BMC Biology (2023)
-
Long term structural and functional neural changes following a single infusion of Ketamine in PTSD
Neuropsychopharmacology (2023)
-
Memory Trace for Fear Extinction: Fragile yet Reinforceable
Neuroscience Bulletin (2023)
-
Reactivating hippocampal-mediated memories during reconsolidation to disrupt fear
Nature Communications (2022)