Abstract
The basal ganglia regulate a wide range of behaviors, including motor control and cognitive functions, and are profoundly affected in Parkinson’s disease (PD). However, the functional organization of different basal ganglia nuclei has not been fully elucidated at the circuit level. In this study, we investigated the functional roles of distinct parvalbumin-expressing neuronal populations in the external globus pallidus (GPe-PV) and their contributions to different PD-related behaviors. We demonstrate that substantia nigra pars reticulata (SNr)-projecting GPe-PV neurons and parafascicular thalamus (PF)-projecting GPe-PV neurons are associated with locomotion and reversal learning, respectively. In a mouse model of PD, we found that selective manipulation of the SNr-projecting GPe-PV neurons alleviated locomotor deficit, whereas manipulation of the PF-projecting GPe-PV neurons rescued the impaired reversal learning. Our findings establish the behavioral importance of two distinct GPe-PV neuronal populations and, thereby, provide a new framework for understanding the circuit basis of different behavioral deficits in the Parkinsonian state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The code that supports the findings of this study is available from the corresponding author upon reasonable request.
References
Graybiel, A. M. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 31, 359–387 (2008).
Nelson, A. B. & Kreitzer, A. C. Reassessing models of basal ganglia function and dysfunction. Annu. Rev. Neurosci. 37, 117–135 (2014).
Kim, J. et al. Inhibitory basal ganglia inputs induce excitatory motor signals in the thalamus. Neuron 95, 1181–1196(2017).
Knowland, D. et al. Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell 170, 284–297 (2017).
Stephenson-Jones, M. et al. A basal ganglia circuit for evaluating action outcomes. Nature 539, 289–293 (2016).
Albin, R. L., Young, A. B. & Penney, J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
Chaudhuri, K. R., Healy, D. G., Schapira, A. H. & National Institute for Clinical, E. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 5, 235–245 (2006).
Kita, H. Globus pallidus external segment. Prog. Brain Res. 160, 111–133 (2007).
Kita, H. & Kitai, S. T. The morphology of globus pallidus projection neurons in the rat: an intracellular staining study. Brain Res. 636, 308–319 (1994).
Hernández, V. M. et al. Parvalbumin+ neurons and Npas1+ neurons are distinct neuron classes in the mouse external globus pallidus. J. Neurosci. 35, 11830–11847 (2015).
Dodson, P. D. et al. Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86, 501–513 (2015).
Mastro, K. J., Bouchard, R. S., Holt, H. A. & Gittis, A. H. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci. 34, 2087–2099 (2014).
Hutchison, W. D. et al. Differential neuronal activity in segments of globus pallidus in Parkinson’s disease patients. Neuroreport 5, 1533–1537 (1994).
Pan, H. S. & Walters, J. R. Unilateral lesion of the nigrostriatal pathway decreases the firing rate and alters the firing pattern of globus pallidus neurons in the rat. Synapse 2, 650–656 (1988).
Filion, M. & Tremblay, L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced Parkinsonism. Brain Res. 547, 142–151 (1991).
Vitek, J. L., Hashimoto, T., Peoples, J., DeLong, M. R. & Bakay, R. A. Acute stimulation in the external segment of the globus pallidus improves Parkinsonian motor signs. Mov. Disord. 19, 907–915 (2004).
Mastro, K. J. et al. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat. Neurosci. 20, 815–823 (2017).
Hikosaka, O., Nakamura, K. & Nakahara, H. Basal ganglia orient eyes to reward. J. Neurophysiol. 95, 567–584 (2006).
Caggiano, V. et al. Midbrain circuits that set locomotor speed and gait selection. Nature 553, 455–460 (2018).
Arkadir, D., Morris, G., Vaadia, E. & Bergman, H. Independent coding of movement direction and reward prediction by single pallidal neurons. J. Neurosci. 24, 10047–10056 (2004).
Brown, H. D., Baker, P. M. & Ragozzino, M. E. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J. Neurosci. 30, 14390–14398 (2010).
Bradfield, L. A., Bertran-Gonzalez, J., Chieng, B. & Balleine, B. W. The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron 79, 153–166 (2013).
Saunders, A., Huang, K. W. & Sabatini, B. L. Globus pallidus externus neurons expressing parvalbumin interconnect the subthalamic nucleus and striatal interneurons. PLoS ONE 11, e0149798 (2016).
Cetin, A. & Callaway, E. M. Optical control of retrogradely infected neurons using drug-regulated ‘TLoop’ lentiviral vectors. J. Neurophysiol. 111, 2150–2159 (2014).
Kato, S. & Kobayashi, K. Improved transduction efficiency of a lentiviral vector for neuron-specific retrograde gene transfer by optimizing the junction of fusion envelope glycoprotein. J. Neurosci. Methods 227, 151–158 (2014).
Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030 (2018).
Pamukcu, A. et al. Parvalbumin+ and Npas1+ pallidal neurons have distinct circuit topology and function. J. Neurosci. 40, 7855–7876 (2020).
Gittis, A. H. et al. New roles for the external globus pallidus in basal ganglia circuits and behavior. J. Neurosci. 34, 15178–15183 (2014).
Abrahao, K. P. & Lovinger, D. M. Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice. J. Physiol. 596, 4219–4235 (2018).
Kim, E. J., Jacobs, M. W., Ito-Cole, T. & Callaway, E. M. Improved monosynaptic neural circuit tracing using engineered rabies virus glycoproteins. Cell Rep. 15, 692–699 (2016).
Saunders, A. et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521, 85–89 (2015).
Broussard, G. J. et al. In vivo measurement of afferent activity with axon-specific calcium imaging. Nat. Neurosci. 21, 1272–1280 (2018).
Kim, C. K. et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods 13, 325–328 (2016).
Lin, J. Y., Lin, M. Z., Steinbach, P. & Tsien, R. Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 (2009).
Birrell, J. M. & Brown, V. J. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 20, 4320–4324 (2000).
Bissonette, G. B. et al. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 28, 11124–11130 (2008).
Blesa, J., Phani, S., Jackson-Lewis, V. & Przedborski, S. Classic and new animal models of Parkinson’s disease. J. Biomed. Biotechnol. 2012, 845618 (2012).
Grospe, G. M., Baker, P. M. & Ragozzino, M. E. Cognitive flexibility deficits following 6-OHDA lesions of the rat dorsomedial striatum. Neuroscience 374, 80–90 (2018).
Oliet, S. H., Malenka, R. C. & Nicoll, R. A. Bidirectional control of quantal size by synaptic activity in the hippocampus. Science 271, 1294–1297 (1996).
Willard, A. M. et al. State transitions in the substantia nigra reticulata predict the onset of motor deficits in models of progressive dopamine depletion in mice. eLife 8, e42746 (2019).
Cools, R., Barker, R. A., Sahakian, B. J. & Robbins, T. W. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex 11, 1136–1143 (2001).
Peterson, D. A. et al. Probabilistic reversal learning is impaired in Parkinson’s disease. Neuroscience 163, 1092–1101 (2009).
Hintiryan, H. et al. The mouse cortico-striatal projectome. Nat. Neurosci. 19, 1100–1114 (2016).
Solari, N., Bonito-Oliva, A., Fisone, G. & Brambilla, R. Understanding cognitive deficits in Parkinson’s disease: lessons from preclinical animal models. Learn. Mem. 20, 592–600 (2013).
Krauss, J. K., Pohle, T., Weigel, R. & Burgunder, J. M. Deep brain stimulation of the centre median-parafascicular complex in patients with movement disorders. J. Neurol. Neurosurg. Psychiatry 72, 546–548 (2002).
Fasano, A., Daniele, A. & Albanese, A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol. 11, 429–442 (2012).
Lim, B. K., Huang, K. W., Grueter, B. A., Rothwell, P. E. & Malenka, R. C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012).
Kato, S. et al. A lentiviral strategy for highly efficient retrograde gene transfer by pseudotyping with fusion envelope glycoprotein. Hum. Gene Ther. 22, 197–206 (2011).
Osakada, F. & Callaway, E. M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 8, 1583–1601 (2013).
Breese, G. R. & Traylor, T. D. Depletion of brain noradrenaline and dopamine by 6-hydroxydopamine. Br. J. Pharmacol. 42, 88–99 (1971).
Park, Y. G. et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol. https://doi.org/10.1038/nbt.4281 (2018).
Tanimura, A., Du, Y., Kondapalli, J., Wokosin, D. L. & Surmeier, D. J. Cholinergic interneurons amplify thalamostriatal excitation of striatal indirect pathway neurons in Parkinson’s disease models. Neuron 101, 444–458 (2019).
McAlonan, K. & Brown, V. J. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 146, 97–103 (2003).
Colacicco, G., Welzl, H., Lipp, H. P. & Würbel, H. Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav. Brain Res. 132, 95–102 (2002).
Bissonette, G. B., Schoenbaum, G., Roesch, M. R. & Powell, E. M. Interneurons are necessary for coordinated activity during reversal learning in orbitofrontal cortex. Biol. Psychiatry 77, 454–464 (2015).
Dhawan, S. S., Tait, D. S. & Brown, V. J. More rapid reversal learning following overtraining in the rat is evidence that behavioural and cognitive flexibility are dissociable. Behav. Brain Res. 363, 45–52 (2019).
Acknowledgements
We thank D. Knowland and C. Santiago for their comments on the manuscript. S. Lilascharoen helped with quantification in Fig. 1 and with illustrations. We thank C. Gremel for essential comments on the reversal learning behavioral experiment. We thank the members of the Lim laboratory for support and discussions. V.L. was supported by the Anandamahidol Foundation Fellowship. This work was supported by grants from the National Institutes of Health (U01NS094342, R01DA049787, R01NS097772, R01MH108594 and U01MH114829).
Author information
Authors and Affiliations
Contributions
V.L. and B.K.L. conceived and designed the study. V.L. performed all electrophysiological recordings. V.L., E.H.W., S.C.P. and A.N.T. performed stereotaxic surgery. V.L., C.D.Y. and E.H.W. performed behavioral experiments. V.L., S.C.P., A.N.T. and X.-Y.W. designed and generated viruses. V.L., J.H.C., N.D., S.C.P. and A.N.T. performed histology and immunohistochemistry. E.H.W. constructed the fiber photometry recording. V.L., S.C.P. and Y.-G.P. performed SHIELD-MAP tissue clearing and light-sheet imaging. Y.-G.P. and K.C. provided resources for SHIELD-MAP. H.P. assisted with the operant behavior experiment and in situ hybridization. V.L., E.H.W. and B.K.L. analyzed the data and interpreted the results. V.L., E.H.W. and B.K.L. wrote the manuscript with contributions from S.C.P. and A.N.T.
Corresponding author
Ethics declarations
Competing interests
K.C. is a co-inventor on a patent application owned by MIT covering the SHIELD technology and is a co-founder of LifeCanvas Technologies.
Additional information
Peer review information Nature Neuroscience thanks Aryn Gittis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Molecular identity of PVGPe-SNr and PVGPe-PF neurons.
a. mFISH experiments for PVGPe-SNr neurons (top row) and PVGPe-PF neurons (bottom row) with several molecular markers for GPe neurons including parvalbumin (Pvalb), somatostatin (Sst), sodium voltage-gated channel beta subunit 4 (Scn4b), LIM homeobox protein (Lhx6), NK2 homeobox 1 (Nkx2-1) and Forkhead box protein P2 (Foxp2). PVGPe-SNr and PVGPe-PF neurons are labelled by the probe against eGFP. Scale bar in a, 20 mm. b, c, Quantification of GFP-labelled PVGPe-SNr neurons (b, n = 3 mice) and GFP-labelled PVGPe-PF neurons (c, n = 4 mice) expressing specific molecular markers. Fractions show the number of neurons expressing each molecular marker out of total GFP positive neurons in sections.
Extended Data Fig. 2 Controls for fiber photometry recordings.
a, Optic fiber placements in PF and SNr for fiber photometry recordings. Different colors denote fibers from the same mouse (n = 7 mice). b, Representative images from n = 7 mice used in photometry recordings showing mRuby3 and axon-GCaMP6s expression in axons of GPe-PV neurons at implantation sites for optic fibers. Scale bars, 200 μm. c, Z-scored ΔF/F (averaged across all events) representing the activity of PVGPe-SNr axons at randomly chosen time points during treadmill locomotion. Number of events was determined based on the number of locomotion onsets in each recording session (n = 135 events). d, same as in c, but showing the activity of PVGPe-PF axons. e, Z-scored ΔF/F (averaged across 7 mice) representing the activity of PVGPe-SNr axons at randomly chosen time points during different stages of reversal-learning task. Number of events was determined based on the number of trials in each stage of the task. f, same as in e, but showing the activity of PVGPe-PF axons. Shaded areas accompanying the z-scored ΔF/F traces in c-f indicate SEM.
Extended Data Fig. 3 Validation of optogenetic activation and chemogenetic inhibition.
a-b, Fiber tip locations in PF (a) and SNr (b) for data in Figs. 4f-g, 5j-k, 7b and Extended Data Fig. 5, 8b-c. c, Representative traces from cell-attached recording showing inhibition of firing activity in SNr neurons during photostimulation of PVGPe-SNr axons at different frequencies. d, Firing rates of SNr neurons during 5-50 Hz and constant photostimulation of PVGPe-SNr axons (n = 21 cells). Data presented as mean ± SEM. e. Representative ex vivo cell-attached recording from PVGPe-SNr neurons (top) and PVGPe-PF neurons (bottom) expressing hM4Di. Gray bars show the application of CNO during recording. f-g. Summary data showing firing rates before and 2 min after CNO bath application of both PVGPe-SNr neurons (f; n = 3 cells; Paired t-test, t(2) = 9.259; *p = 0.0115) and PVGPe-PF neurons (g; n = 3 cells; Paired t-test, t(2) = 5.459; *p = 0.0320).
Extended Data Fig. 4 Structure of the reversal-learning task and comparison of overall neural activity during trials and inter-trial intervals.
a, Example task structure using gravel and sand as digging media to provided two different contexts. The food reward is paired with sand in the association phase and is later switched to gravel in the reversal-learning phase. b, Timeline of the task showing how the association, early, and late reversal-learning stages are defined for a representative mouse. Check marks and crosses represent correct and incorrect trials, respectively. c, Average speed of animals across different stages of the reversal learning task. Note that no significant difference in locomotion was observed at different stages of the task. (Left: Duration of Trial to Choice, n = 9 mice, One-way ANOVA, F(2, 24) = 0.4743, p = 0.6280; Right: Duration of Choice, n = 9, One-way ANOVA, F(2, 24) = 0.7019, p = 0.5055). All data presented as mean ± SEM. d, Comparison of mean z-scored ΔF/F for PVGPe-SNr axons between trial periods and inter-trial intervals (ITI) during the association phase (left; Wilcoxon sign-rank test, W = -12; p = 0.3750, n = 7 mice) and reversal-learning phase (right; Wilcoxon sign rank test, W = -18; p = 0.1562, n = 7 mice). e, same as in d, but for PVGPe-PF axons during the association phase (left; Wilcoxon sign-rank test, W = -28; *p = 0.0156, n = 7 mice) and reversal-learning phase (right; Wilcoxon sign rank test, W = -28; *p = 0.0156, n = 7 mice).
Extended Data Fig. 5 Activation of PVGPe-PF neurons increased number of regressive errors made during reversal learning.
a-b, Number of errors during the reversal-learning phase made by mice that received photostimulation in PVGPe-SNr neurons (n = 7 mice for eGFP, n = 9 mice for oChIEF). a, Perseverative errors; Unpaired t-test, t(14) = 0.9432, p = 0.3616. b, Regressive errors; Unpaired t-test, t(14) = 0.3002, p = 0.7684. c-d, Number of errors during the reversal-learning phase made by mice that received photostimulation in PVGPe-PF neurons (n = 10 mice for eGFP, n = 8 mice for oChIEF). c, Perseverative errors; Unpaired t-test, t(16) = 0.8109, p = 0.4293. d, Regressive errors; Unpaired t-test, t(16) = 2.951, **p = 0.0094. All data presented as mean ± SEM.
Extended Data Fig. 6 Role of PVGPe-SNr and PVGPe-PF in reversal learning test for discriminated operant response in lever-pressing system.
a, A schematic diagram for behavioral task. After the rule switch, active lever becomes inactive, and vice versa. b-d, Traces of z-scored ΔF/F (averaged across 5 mice) from PVGPe-PF axons during session start (b), from start to lever pressing (c) and during lever pressing (d) at the different behavioral stage. Note that the fiber photometry signals for interval between session start and lever pressing was interpolated because of the difference in interval. e-g, Traces of z-scored ΔF/F (averaged across 4 mice) from PVGPe-SNr axons during session start (e), from start to lever pressing (f) and during lever pressing (g) at the different behavioral stage. Note that the fiber photometry signals for interval between session start and lever pressing was interpolated because of the difference in interval. h-k, Activation of PVGPe-SNr and PVGPe-PF axons did not affect association (h, j) and reversal (i, k) in operant discrimination tasks. l-o, Inhibition of PVGPe-SNr and PVGPe-PF axons did not affect association (l, n) and reversal (m, o) in operant discrimination tasks. Shaded areas accompanying the z-scored ΔF/F traces in b-g indicate SEM. All other data are presented as mean ± SEM.
Extended Data Fig. 7 Quantification of TH immunoreactivity.
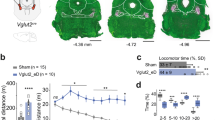
a, Representative image of TH immunoreactivity in the striatum of a mouse injected with vehicle (0.02% sodium ascorbate in 0.9% saline). b, Representative image of TH immunoreactivity in the striatum 3 days after bilateral injection of low-dose 6-OHDA (1.25 μg/μl). c, Representative image of TH immunoreactivity in the striatum 10 days after bilateral injection of high-dose 6-OHDA (2.5 μg/μl). d, Quantification of TH immunoreactivity at different stages of dopamine depletion in rescue experiments for reversal learning and locomotion. Data presented as % mean ± SEM of naïve control striatal sections (n = 6 mice for PVGPe-PF: eGFP (vehicle), n = 7 mice for PVGPe-PF: eGFP (OHDA), n = 7 mice for PVGPe-PF: hM4D (OHDA), n = 10 mice for PVGPe-SNr: eGFP (OHDA), and n = 11 mice for PVGPe-SNr: oChIEF (OHDA)). Scale bar, 1 mm (a-c).
Extended Data Fig. 8 Manipulation of PVGPe-PF but not PVGPe-SNr neurons rescues behavioral flexibility deficit in dopamine-depleted mice.
a, Number of errors during the reversal-learning phase made by mice that received chemogenetic inhibition in PVGPe-PF neurons after dopamine depletion (n = 7 mice for eGFP-vehicle, n = 7 mice for eGFP-OHDA, and n = 7 mice for hM4Di-OHDA). Left, perseverative errors; One-way ANOVA, F(2,18) = 0.9771, p = 0.3955. Right, regressive errors; One-way ANOVA, F(2,18) = 5.595, p = 0.0129; Bonferroni’s post hoc test, *p = 0.0312 (eGFP-vehicle vs. eGFP-OHDA) and 0.0267 (eGFP-OHDA vs. hM4Di-OHDA). b, Performance of mice that received photostimulation in PVGPe-SNr neurons after dopamine depletion (n = 6 mice for eGFP-vehicle, n = 4 mice for eGFP-OHDA, and n = 6 mice for oChIEF-OHDA). Left, dopamine depletion did not affect performance in the association phase. One-way ANOVA, F(2,13) = 0.8222, p = 0.4611. Right, activation of PVGPe-SNr neurons during reversal learning did not improved behavioral flexibility in dopamine-depleted mice. One-way ANOVA, F(2,13) = 11.69, p = 0.0012; Bonferroni’s post hoc test, **p = 0.0032 (eGFP-vehicle vs. eGFP-OHDA) and 0.0042 (eGFP-vehicle vs. oChIEF-OHDA). c, Number of errors during the reversal-learning phase made by mice in c. Left, perseverative errors; One-way ANOVA, F(2,13) = 1.308, p = 0.3038. Right, regressive errors; One-way ANOVA, F(2,13) = 0.6464, p = 0.54. All data presented as mean ± SEM.
Supplementary information
Rights and permissions
About this article
Cite this article
Lilascharoen, V., Wang, E.HJ., Do, N. et al. Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits. Nat Neurosci 24, 504–515 (2021). https://doi.org/10.1038/s41593-021-00810-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-021-00810-y
This article is cited by
-
Updating the striatal–pallidal wiring diagram
Nature Neuroscience (2024)
-
The longitudinal volumetric and shape changes of subcortical nuclei in Parkinson’s disease
Scientific Reports (2024)
-
Cell and circuit complexity of the external globus pallidus
Nature Neuroscience (2023)
-
A non-canonical striatopallidal Go pathway that supports motor control
Nature Communications (2023)
-
Angiotensin II increases the firing activity of pallidal neurons and participates in motor control in rats
Metabolic Brain Disease (2023)