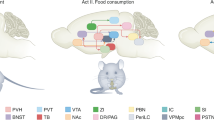
Abstract
Maintaining healthy body weight is increasingly difficult in our obesogenic environment. Dieting efforts are often overpowered by the internal drive to consume energy-dense foods. Although the selection of calorically rich substrates over healthier options is identifiable across species, the mechanisms behind this choice remain poorly understood. Using a passive devaluation paradigm, we found that exposure to high-fat diet (HFD) suppresses the intake of nutritionally balanced standard chow diet (SD) irrespective of age, sex, body mass accrual and functional leptin or melanocortin-4 receptor signaling. Longitudinal recordings revealed that this SD devaluation and subsequent shift toward HFD consumption is encoded at the level of hypothalamic agouti-related peptide neurons and mesolimbic dopamine signaling. Prior HFD consumption vastly diminished the capacity of SD to alleviate the negative valence associated with hunger and the rewarding properties of food discovery even after periods of HFD abstinence. These data reveal a neural basis behind the hardships of dieting.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data and supporting materials will be made available by the corresponding authors upon reasonable request.
Code availability
Code is available by the corresponding authors upon request or directly at https://www.niehs.nih.gov/research/atniehs/labs/ln/pi/iv/tools/index.cfm.
References
Timper, K. & Brüning, J. C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model. Mech. 10, 679–689 (2017).
DiFeliceantonio, A. G. & Small, D. M. Dopamine and diet-induced obesity. Nat. Neurosci. 22, 1–2 (2019).
Ferrario, C. R. et al. Homeostasis meets motivation in the battle to control food intake. J. Neurosci. 36, 11469–11481 (2016).
Luquet, S., Perez, F. A., Hnasko, T. S. & Palmiter, R. D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Takahashi, K. A. & Cone, R. D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide y/agouti-related protein neurons. Endocrinology 146, 1043–1047 (2005).
Mandelblat-Cerf, Y. et al. Arcuate hypothalamic AgRP and putative pomc neurons show opposite changes in spiking across multiple timescales. eLife 4, 1–25 (2015).
Chen, Y., Lin, Y.-C., Kuo, T.-W. & Knight, Z. A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015).
Beutler, L. R. et al. Dynamics of gut–brain communication underlying hunger. Neuron 96, 461–475 (2017).
Su, Z., Alhadeff, A. L. & Betley, J. N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep. 21, 2724–2736 (2017).
Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015).
Baver, S. B. et al. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 34, 5486–5496 (2014).
Salamone, J. D., Correa, M., Mingote, S. & Weber, S. M. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharmacol. Exp. Ther. 305, 1–8 (2003).
Berridge, K. C. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol. Behav. 97, 537–550 (2009).
Wise, R. A. Role of brain dopamine in food reward and reinforcement. Philos. Trans. R. Soc. B Biol. Sci. 361, 1149–1158 (2006).
Alhadeff, A. L. et al. Natural and drug rewards engage distinct pathways that converge on coordinated hypothalamic and reward circuits. Neuron 103, 891–908.e6 (2019).
Denis, R. G. P. et al. Palatability can drive feeding independent of AgRP neurons. Cell Metab. 22, 646–657 (2015).
Fordahl, S. C. & Jones, S. R. High-fat-diet-induced deficits in dopamine terminal function are reversed by restoring insulin signaling. ACS Chem. Neurosci. 8, 290–299 (2017).
Rothemund, Y. et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37, 410–421 (2007).
Stice, E., Spoor, S., Bohon, C., Veldhuizen, M. G. & Small, D. M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol. 117, 924–935 (2008).
Thanarajah, S. E. et al. Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans. Cell Metab. 29, 695–706 (2019).
Ravussin, Y. et al. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am. J. Physiol. Integr. Comp. Physiol. 300, R1352–R1362 (2011).
Johnson, P. M. & Kenny, P. J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 13, 635–641 (2010).
Cone, J. J., Chartoff, E. H., Potter, D. N., Ebner, S. R. & Roitman, M. F. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS ONE 8, e58251 (2013).
Drewnowski, A. & Greenwood, M. R. C. Cream and sugar: human preferences for high-fat foods. Physiol. Behav. 30, 629–633 (1983).
Yang, Y. Jr, D. L, S., Keating, K. D., Allison, D. B. & Nagy, T. R. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity 22, 2147–2155 (2014).
Guo, J., Jou, W., Gavrilova, O. & Hall, K. D. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PLoS ONE 4, e5370 (2009).
Carlin, J. L. et al. Removal of high-fat diet after chronic exposure drives binge behavior and dopaminergic dysregulation in female mice. Neuroscience 326, 170–179 (2016).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Jais, A. & Brüning, J. C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest. 127, 24–32 (2017).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Garfield, A. S. et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 19, 1628–1635 (2016).
Hahn, T. M., Breininger, J. F., Baskin, D. G. & Schwartz, M. W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1, 271–272 (1998).
Briggs, D. I., Enriori, P. J., Lemus, M. B., Cowley, M. A. & Andrews, Z. B. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 151, 4745–4755 (2010).
Briggs, D. I. et al. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology 154, 709–717 (2013).
Liu, S. et al. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proc. Natl Acad. Sci. USA 113, 2520–2525 (2016).
Roitman, M. F., Stuber, G. D., Phillips, P. E. M., Wightman, R. M. & Carelli, R. M. Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 24, 1265–1271 (2004).
Atasoy, D., Aponte, Y., Su, H. H. & Sternson, S. M. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Alexander, G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009).
Tsai, H.-C. et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324, 1080–1084 (2009).
Sun, F. et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 174, 481–496 (2018).
Betley, J. N., Cao, Z. F. H., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Lippert, R. N. et al. Maternal high-fat diet during lactation reprograms the dopaminergic circuitry in mice. J. Clin. Invest. 130, 3761–3776 (2020).
Tellez, L. A. et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat. Neurosci. 19, 465–470 (2016).
Stice, E., Yokum, S., Blum, K. & Bohon, C. Weight gain is associated with reduced striatal response to palatable food. J. Neurosci. 30, 13105–13109 (2010).
Volkow, N. D., Wang, G.-J. & Baler, R. D. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 15, 37–46 (2011).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Meng, C. et al. Spectrally resolved fiber photometry for multi-component analysis of brain circuits. Neuron 98, 707–717 (2018).
Nguyen, K. P., O’Neal, T. J., Bolonduro, O. A., White, E. & Kravitz, A. V. Feeding Experimentation Device (FED): a flexible open-source device for measuring feeding behavior. J. Neurosci. Methods 267, 108–114 (2016).
Acknowledgements
Research was supported by an NIEHS–NIDDK joint fellowship. We thank N. Martin and B. Gloss of the NIEHS Viral Vector Core for producing AAVs and J. Tucker of the NIEHS Fluorescence Microscopy and Imaging Center for assistance with image acquisition. We thank the GENIE project for the development of GCaMP6. We would also like to thank all members of Dr. Krashes’ and Dr. Cui’s labs for their technical support and guidance throughout this work and J. Cushman of the NIEHS Neurobehavioral Core for assistance with behavioral studies and statistical analyses. This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (1ZIAES103310 to G.C.), the National Institutes of Diabetes and Digestive and Kidney Diseases (DK075088 to M.J.K. and DK075087-06 to M.J.K.), the NIEHS–NIDDK Joint Fellowship Award (to C.M.M.) and the Center of Compulsive Behaviors (to C.M.M. and I.D.A.S.).
Author information
Authors and Affiliations
Contributions
C.M.M., J. L.-G. and M.J.K. designed experiments with technical input from G.C., J. L.-G., N.S.W. and M.H.B. M.S. performed and analyzed home cage consumption and body composition, fast–refeed and optogenetic experiments. C.M.M. performed and analyzed home cage AgRP fiber photometry experiments and DA sensor experiments. C.L. performed and analyzed LepR-Cre fiber photometry experiments. C.L. and I.D.A.S. performed electrophysiology recordings. J. L.-G. performed and analyzed gastric infusion and VTA studies. F.S., Y.Z. and Y.L. provided the DA2m sensor and provided technical guidance. N.P.K. and D.M.R. performed histological verification and imaging. C.M.M., J. L.-G. and M.J.K. wrote the manuscript with input from G.C., C.L., I.D.A.S., N.S.W., M.H.B., M.S., N.P.K., D.M.R., F.S, Y.Z. and Y.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Paul Kenny and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 HFD-exposure promotes fat mass accrual, reduction of homecage SD intake and devaluation of SD in physiologically hungry mice independent of weight gain.
Weekly a, fat mass (n = 20, males and females, RM two-way ANOVA, Week x Group: F (11, 418) = 50.17, P < 0.0001, Sidak’s multiple comparisons test) and b, lean mass throughout diet exposure (n = 20, males and females, RM two-way ANOVA, Week x Group: F (11, 418) = 0.8075, P = 0.6326). Daily c, body weights and d, SD intake throughout the duration of the experiment of individual animals exposed to HFD. e, No correlation between the average amount of SD intake during the HFD-exposure period and body weight changes after 8 weeks of SD and HFD access (n = 20, males and females, Linear regression, R2 = 0.02713, P = 0.4877). f, Between-subject comparison of 1 hr SD fast-refeed consumption across test sessions (n = 16 SD group, n = 18 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (4, 128) = 28.99, P < 0.0001, Bonferroni’s multiple comparisons test). g, No correlation between SD devaluation at Week 8 relative to Baseline and body weight changes after 8 weeks of access to SD and HFD (n = 18, males and females, Linear regression, R2=0.001090, P = 0.8965). h, Experimental timeline, group schematic for home cage measurements and i, and daily body weights throughout the duration of the experiment (n = 12 per group, males and females). j, Within-subject comparison of 1 hr SD fast-refeed consumption across testing sessions (n = 12 limited HFD weight-matched to SD group, males and females, RM one-way ANOVA, Time: F (2.431, 26.74) = 66.81, P < 0.0001, Tukey’s multiple comparisons). k, Within-subject, within-diet comparisons of 1 hr SD and HFD fast-refeed consumption across testing sessions (n = 18, males and females, RM one-way ANOVAs, Tukey’s multiple comparisons). Dotted lines in a–d and i delineate window of HFD availability. All error bars and shaded areas in a, b and i represent mean ± s.e.m. Shaded blue area in f, j, and k represent HFD homecage availability. *P < 0.05, **P < 0.01, ****P < 0.0001.
Extended Data Fig. 2 Intact melanocortin-4 receptor- or leptin-signaling are dispensable for SD devaluation following HFD-exposure and 24 h of HFD-exposure is sufficient to devalue SD.
a, d Experimental timeline and group schematic for fast-refeed test with 1 hr SD access. b, e Within-subject and c, f between-subject comparisons of 1 hr SD fast-refeed consumption across testing sessions (n = 5 per Mc4r KO + /- and -/- group, males and females, RM two-way ANOVA, Time x Group: F (4, 32) = 0.3173, P = 0.8643, Tukey’s multiple comparisons) (n = 4 per Leptin KO + /- and -/- group, males and females, RM two-way ANOVA, Time x Group: F (4, 24) = 0.3313, P = 0.8542, Tukey’s multiple comparisons). g, Experimental timeline and group schematic for fast-refeed test with 1 hr SD access. h, Within-subject and i, between-subject comparisons of 1 hr SD fast-refeed consumption across testing sessions (n = 16 per group, males and females, RM two-way ANOVA, Time x Group: F (1, 30) = 16.96, P = 0.0003, Sidak’s multiple comparisons test). B = Baseline. WD = withdrawal. Shaded blue area in b, c, e, f, h and i represent HFD homecage availability. All error bars represent s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 3 Acute and chronic HFD-exposure alters ARCAgRP activity responses to SD and HFD in fasted mice largely independent of body-weight changes.
a, Between-subject quantification of fasted ARCAgRP activity response to Pellet 1 (SD) (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 16.74, P < 0.0001, Bonferroni’s multiple comparisons) and b, SD consumed across testing sessions (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 15.81, P < 0.0001, Bonferroni’s multiple comparisons). c–f, ARCAgRP activity changes to Pellet 1 (SD) presentation are not strongly correlated with bodyweight accrual over the entire length of the experiment (n = 12 SD group, n = 11 SD + HFD group, males and females, Linear regression, c, SD group: R2 = 0.001058, P = 0.9201, SD + HFD group: R2 = 0.6731, P = 0.0020, d, SD group: R2 = 0.2304, P = 0.1142, SD + HFD group: R2 = 0.1154, P = 0.3067, e, SD group: R2 = 0.1539, P = 0.2072, SD + HFD group: R2 = 0.1319, P = 0.2723, f, SD group: R2 = 0.002643, P = 0.8739, SD + HFD group: R2 = 0.2958, P = 0.0837) g, Between-subject quantification of fasted ARCAgRP activity response to Pellet 2 (SD for SD group; SD for SD + HFD group during B1 and B2 and HFD for SD + HFD group during 1, 4, 8 and 10 weeks) (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 21.07, P < 0.0001, Bonferroni’s multiple comparisons) and h, total calories consumed across testing sessions (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 14.26, P < 0.0001, Bonferroni’s multiple comparisons). i, Experimental timeline and group schematic for fasted photometry recordings. j, Average fasted photometry traces across recording sessions aligned to Pellet 1 and 2 presentation (n = 6, males and females). k, Within-subject quantification of fasted ARCAgRP activity response to Pellet 1 (SD) (n = 6, males and females, Paired t test (two-tailed), P = 0.0099) and l, SD consumed across testing sessions (n = 6, males and females, Paired t test (two-tailed), P = 0.0049). m, Within-subject quantification of fasted ARCAgRP activity response to Pellet 2 (SD for Baseline; HFD for 1 day) (n = 6, males and females, Paired t test (two-tailed), P = 0.0008) and n, total calories consumed across testing sessions (n = 6, males and females, Paired t test (two-tailed), P = 0.0008). Shaded blue area in a, b, and g, h represent HFD homecage availability. B1 and B2 refer to Baseline 1 and 2, respectively. WD = withdrawal. Dotted lines in j indicate Pellet 1 and Pellet 2 presentation. All error bars and shaded regions of j represent s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 4 HFD-exposure alters ARCAgRP activity responses to HFD in fed mice.
a, Experimental timeline and group schematic for fed photometry recordings. b, c, Average fed photometry traces of the b, SD group and c, SD + HFD group across recording sessions aligned to Pellet 1 and 2 presentation (n = 12 SD group, n = 11 SD + HFD group, males and females). d, Within- and e, between-subject quantification of fed ARCAgRP activity response to Pellet 1 (SD) (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 0.8953, P = 0.4872, Bonferroni’s multiple comparisons). f, Within- and g, between-subject quantification of SD consumed across testing sessions (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 1.768, P = 0.1258). h, Within- and i, between-subject quantification of fed ARCAgRP activity response to Pellet 2 (SD for SD group; SD for SD + HFD group during B1 and B2 and HFD for SD + HFD group during 1, 4, 8 and 10 weeks) (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 7.565, P < 0.0001, Bonferroni’s multiple comparisons). j, Within- and k, between-subject quantification of total calories consumed across testing sessions (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 31.68, P < 0.0001, Bonferroni’s multiple comparisons). Dotted lines in b, c indicate Pellet 1 and Pellet 2 presentation. Shaded blue area in d–k represent HFD homecage availability. B1 and B2 refer to Baseline 1 and 2, respectively. WD = withdrawal. All error bars and shaded regions of b, c represent s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 5 Spectrum-based fiber photometry reveals diminished ARCAgRP baseline activity following HFD exposure.
a, Representative GCaMP and tdTomato emission spectra from a mouse during the Fed and Fasted sessions of Baseline 1 prior to the addition of food. b, GCaMP/tdTomato ratios normalized to the mean of Baseline 1 + 2 Fed values shows basal ARCAgRP activity is increased during Fasted sessions relative to Fed sessions (SD – Fed vs Fasted; HFD – Fed vs Fasted). Homecage HFD exposure reduces basal ARCAgRP activity relative to the SD group across both Fed and Fasted conditions (Fed – SD vs HFD; Fasted – SD vs HFD) (n = 12 SD group, n = 11 SD + HFD group, males and females, three-way ANOVA, Diet: F(1,21) = 6.515, P = 0.019, Satiety state: F(1,63) = 94.128, P < 0.0001) c, Fasted GCaMP/tdTomato ratios normalized to the Fed values across recording sessions demonstrates an ~50% fasting-induced increase in the GCaMP/tdTomato ratio across all recording weeks (n = 12 SD group, n = 11 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (5, 105) = 1.978, P = 0.0879). B1 and B2 refer to Baseline 1 and 2, respectively. WD = withdrawal.
Extended Data Fig. 6 Physiological disruption of ARCAgRP activity in response to HFD-exposure is partially dependent on body weight gain.
a, Changes in ARCAgRP activity in response to identical caloric load is correlated to weight gain 1, but not 4 weeks after HFD exposure or 1 week HFD withdrawal (n = 6, males and females, Linear regression, 1 week (HFD): R2 = 0.6903, P = 0.0405, 4 weeks (HFD): R2 = 0.1356, P = 0.4727, 5 weeks (WD): R2 = 0.003755, P = 0.9082). b-e, Changes in ARCAgRP activity in response to ghrelin is correlated to weight gain (n = 12 SD group, n = 11 SD + HFD group, males and females, Linear regression, b, SD group: R2 = 0.01466, P = 0.7078, SD + HFD group: R2 = 0.3905, P = 0.0398, c, SD group: R2 = 0.05629, P = 0.4578, SD + HFD group: R2 = 0.4724, P = 0.0194, d, SD group: SD group: R2 = 0.1504, P = 0.2129, SD + HFD group: R2 = 0.2336, P = 0.1321), e, SD group: R2 = 0.2907, P = 0.0705, SD + HFD group: R2 = 0.4042, P = 0.0355. f–k, Changes in ARCAgRP activity in response to f, g, CCK, h–I, 5HT or j, k, PYY is not correlated to weight gain (n = 5 SD group, n = 6 SD + HFD group, males and females, Linear regression, f, SD group: R2 = 0.008391, P = 0.8835, SD + HFD group: R2 = 0.09392, P = 0.5547, g, SD group: R2 = 0.003959, P = 0.9199, SD + HFD group: R2 = 0.06259, P = 0.6326, h, SD group: R2 = 0.6037, P = 0.1221, SD + HFD group: R2 = 0.0009157, P = 0.9546, i, SD group: R2 = 0.6415, P = 0.1034, SD + HFD group: R2 = 0.0007729, P = 0.9583, j, SD group: R2 = 0.3514, P = 0.2922, SD + HFD group: R2 = 0.05138, P = 0.6602, k, SD group: R2 = 0.09218, P = 0.6194, SD + HFD group: R2 = 0.01706, P = 0.8052). l, n, p, Average photometry traces of the SD group across recording sessions aligned to l, CCK, n, 5HT or p, PYY injection (n = 5 SD group, males and females). m, o, q, Within-subject quantification of ARCAgRP activity to m, CCK (n = 5 SD group, males and females, RM two-way ANOVA, Time Bin x Week: F (1.511, 6.045) = 1.014, P = 0.3923), o, 5HT (n = 5 SD group, males and females, RM two-way ANOVA, Time Bin x Week: F (2.334, 9.337) = 1.242, P = 0.3393) or q, PYY (n = 5 SD group, males and females, RM two-way ANOVA, Time Bin x Week: F (1.897, 7.589) = 1.885, P = 0.2164) across testing sessions. Dotted lines indicate l, CCK, n, 5HT or p, PYY injection. B1 and B2 refer to Baseline 1 and 2, respectively. All error bars and shaded regions of l, n, and p represent s.e.m.
Extended Data Fig. 7 HFD exposure promotes devaluation of optogenetic ARCAgRP-evoked SD, but not HFD, and consumption independent of weight gain.
a, Between-subject comparison of fedAgRP stimulation 1 hr SD consumption across test sessions (n = 16 SD group, n = 17 SD + HFD group, males and females, RM two-way ANOVA, Time x Group: F (4, 124) = 18.85, P < 0.0001, Bonferroni’s multiple comparisons). b, No correlation between SD devaluation at Week 8 relative to Baseline and body weight changes after 8 weeks of access to SD and HFD (n = 17, males and females, Linear regression, R2 = 0.04964, P = 0.3900). c, Within-subject, within-diet comparisons of fedAgRP stimulation 1 hr SD and HFD consumption across testing sessions (n = 17, males and females, RM one-way ANOVAs, Tukey’s multiple comparisons). B = Baseline. WD = Withdrawal. Shaded blue area in a and c represent HFD homecage availability. All error bars represent s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 8 ARCAgRP/Npy activation does not alter basal VTADat activity but potentiates signaling in response to food via an indirect signaling mechanism.
a, Brain schematic of anterograde tracing strategy. b–d, representative images of AgRP::Synaptophysin-tdTomato and tyrosine hydroxylase (TH) in the b, arcuate nucleus, c, paraventricular hypothalamus and d, ventral tegmental area. e, Brain schematic of unilateral viral delivery of Cre-inducible ChR2 to the ARC of AgRP-ires-Cre mice and position of ex vivo slice recordings from PVH (top) and VTA (bottom) cells. f, g, ChR2-assisted circuit mapping schematic and representative trace from f, PVH and g, VTA cells. h, Representative image of Dat-ires-Cre::Ai32-ChR2-eYFP and overlap with TH in the ARC. i, Chemogenetic ARCNpy activation drives food intake (n = 6, males and females, Paired t test (two-tailed), P = 0.0013). j, Averaged Z-score traces and k, quantification of basal VTADat activity after saline or CNO injections (n = 6, males and females, Paired t test (two-tailed), P = 0.2837). l, Averaged Z-score traces and m, mean (n = 6, males and females, Paired t test (two-tailed), P = 0.0656) and n, max quantification (n = 6, males and females, Paired t test (two-tailed), P = 0.0365) of VTADat activity in response to SD food drop after saline or CNO injections. All error bars represent s.e.m. *P < 0.05, **P < 0.01.
Extended Data Fig. 9 VTADat photostimulation is intrinsically rewarding but loses the capacity to enhance SD consumption in fasted mice after HFD exposure.
a, Schematic of Real-Time Place Preference assay. b, Averaged heat maps and quantification of time spent in each chamber of the RTPP assay in VTADat::ChR2 mice (n = 9, males, Paired t test (two-tailed), P < 0.0001). c, Averaged heat maps and quantification of time spent in each chamber of the RTPP assay in control VTADat::YFP mice (n = 6, males, Paired t test (two-tailed), P = 0.9576). d, Within-subject and condition comparisons of 1 hr SD consumption across testing sessions with or without VTADat photostimulation (n = 9, males, RM two-way ANOVA, Time x Condition: F (3, 48) = 18.30, P < 0.0001, Tukey’s multiple comparisons). B = Baseline. WD = Withdrawal. Shaded blue area in d represents HFD homecage availability. All error bars represent s.e.m. ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 10 DA responses to SD are state-dependent and consistent over time in HFD-naïve animals.
a, Averaged Z-score traces of DA2m fluorescence aligned to SD pellet retrieval across appetite state (n = 8, males and females). b, Within-subject comparisons of mean fluorescence Z-score during SD pellet retrieval across appetite state (n = 8, males and females, Paired t test (two-tailed), P = 0.0659). c, Within-subject comparisons of SD pellets consumed across appetite state (n = 8, males and females, Paired t test (two-tailed), P < 0.0001). d, Averaged Z-score traces of DA2m fluorescence aligned to SD pellet retrieval across recording weeks. (n = 4, males and females) e, Within-subject comparisons of mean fluorescence Z-score during SD pellet retrieval across sessions (n = 4, males and females, Wilcoxon matched-pairs signed rank test, P = 0.625). f, Within-subject comparisons of SD pellets consumed across sessions (n = 4, males and females, Wilcoxon matched-pairs signed rank test, P > 0.9999). Time 0 denotes consumption. B = Baseline. Shaded peach region in a and d represent quantified pellet approach/retrieval period. All error bars and shaded regions of a and d represent s.e.m. ****P < 0.0001.
Supplementary information
Supplementary Table 1
Supplementary Table 1. Statistics.
Rights and permissions
About this article
Cite this article
Mazzone, C.M., Liang-Guallpa, J., Li, C. et al. High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nat Neurosci 23, 1253–1266 (2020). https://doi.org/10.1038/s41593-020-0684-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0684-9
This article is cited by
-
Protection against overfeeding-induced weight gain is preserved in obesity but does not require FGF21 or MC4R
Nature Communications (2024)
-
ANMCO (Italian Association of Hospital Cardiologists) scientific statement: obesity in adults—an approach for cardiologists
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2024)
-
Konservative Adipositastherapie
Die Diabetologie (2024)
-
The metabolic effects of resumption of a high fat diet after weight loss are sex dependent in mice
Scientific Reports (2023)
-
Early central cardiovagal dysfunction after high fat diet in a murine model
Scientific Reports (2023)