Abstract
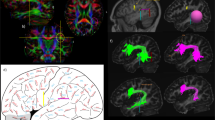
The human arcuate fasciculus pathway is crucial for language, interconnecting posterior temporal and inferior frontal areas. Whether a monkey homolog exists is controversial and the nature of human-specific specialization unclear. Using monkey, ape and human auditory functional fields and diffusion-weighted MRI, we identified homologous pathways originating from the auditory cortex. This discovery establishes a primate auditory prototype for the arcuate fasciculus, reveals an earlier phylogenetic origin and illuminates its remarkable transformation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The ultra-high resolution postmortem macaque dataset is from a previously shared resource. The chimpanzee and human datasets are from available resources (S500 group of the Human Connectome Project, http://www.chimpanzeebrain.org; the chimpanzee resource is available upon request and the NCBR staff are contacted via the main landing page). The awake macaque dMRI data is available from the Open Science Framework at https://osf.io/arqp8/ and will be made available at the PRIMatE MRI Data Exchange (https://fcon_1000.projects.nitrc.org/indi/indiPRIME.html). The ROIs are found in available atlases or as part of previous publications.
Code availability
Custom code and analyses were not required. Analysis pipelines used FSL (FDT v.6.0.1); other processing pipelines are as noted.
References
Hagoort, P. The neurobiology of language beyond single-word processing. Science 366, 55–58 (2019).
Anwander, A., Tittgemeyer, M., von Cramon, D. Y., Friederici, A. D. & Knösche, T. R. Connectivity-based parcellation of Broca’s area. Cereb. Cortex 17, 816–825 (2007).
Price, C. J., Seghier, M. L. & Leff, A. P. Predicting language outcome and recovery after stroke: the PLORAS system. Nat. Rev. Neurol. 6, 202–210 (2010).
Bornkessel-Schlesewsky, I., Schlesewsky, M., Small, S. L. & Rauschecker, J. P. Neurobiological roots of language in primate audition: common computational properties.Trends Cogn. Sci. 19, 142–150 (2015).
Skeide, M. A. & Friederici, A. D. Response to Bornkessel-Schlesewsky et al.—Towards a nonhuman primate model of language? Trends Cogn. Sci. 19, 483 (2015).
Thiebaut de Schotten, M., Dell’Acqua, F., Valabregue, R. & Catani, M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48, 82–96 (2012).
Rilling, J. K. et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 11, 426–428 (2008).
Eichert, N. et al. What is special about the human arcuate fasciculus? Lateralization, projections, and expansion. Cortex 118, 107–115 (2019).
Frey, S., Mackey, S. & Petrides, M. Cortico-cortical connections of areas 44 and 45B in the macaque monkey. Brain Lang. 131, 36–55 (2014).
Rilling, J., Glasser, M. F., Jbabdi, S., Andersson, J. & Preuss, T. M. Continuity, divergence, and the evolution of brain language pathways. Front. Evol. Neurosci. 3, 11 (2012).
Mars, R. B., Eichert, N., Jbabdi, S., Verhagen, L. & Rushworth, M. F. S. Connectivity and the search for specializations in the language-capable brain. Curr. Opin. Behav. Sci. 21, 19–26 (2018).
Calabrese, E. et al. A diffusion tensor MRI atlas of the postmortem rhesus macaque Brain. Neuroimage 117, 408–416 (2015).
Schmahmann J. D. & Pandya D. N. Fiber Pathways of the Brain (Oxford Univ. Press, 2009).
Bailey, P., Von Bonin, G., Garol, H. W. & McCulloch, W. S. Functional organization of temporal lobe of monkey (Macaca mulatta) and chimpanzee (Pan satyrus). J. Neurophysiol. 6, 121–128 (1943).
Takaya, S. et al. Asymmetric projections of the arcuate fasciculus to the temporal cortex underlie lateralized language function in the human brain. Front. Neuroanat. 9, 119 (2015).
Romanski, L. M. et al. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 2, 1131–1136 (1999).
Rauschecker, J. P. & Scott, S. K. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat. Neurosci. 12, 718–724 (2009).
Zhang, Y. S. & Ghazanfar, A. A. A hierarchy of autonomous systems for vocal production. Trends Neurosci. 43, P115–P126 (2020).
Wilson, B. et al. Auditory sequence processing reveals evolutionarily conserved regions of frontal cortex in macaques and humans. Nat. Commun. 6, 8901 (2015).
Flinker, A. & Knight, R. T. Broca’s area in comprehension and production, insights from intracranial studies in humans. Curr. Opin. Behav. Sci. 21, 170–175 (2018).
Petkov, C. I., Kayser, C., Augath, M. & Logothetis, N. K. Functional imaging reveals numerous fields in the monkey auditory cortex. PLoS Biol. 4, e215 (2006).
Baumann, S. et al. Characterisation of the BOLD response time course at different levels of the auditory pathway in non-human primates. Neuroimage 50, 1099–1108 (2010).
van Essen, D. C. et al. The Human Connectome Project: a data acquisition perspective. Neuroimage 62, 2222–2231 (2012).
Dick, F. et al. In vivo functional and myeloarchitectonic mapping of human primary auditory areas. J. Neurosci. 32, 16095–16105 (2012).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004).
Behrens, T. E. J. et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn. Reson. Med. 50, 1077–1088 (2003).
Zhang, Y., Brady, M. & Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57 (2001).
Hackett, T. A., Preuss, T. M. & Kaas, J. H. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J. Comp. Neurol. 441, 197–222 (2001).
Tournier, J.-D., Calamante, F. & Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35, 1459–1472 (2007).
Acknowledgements
We thank N. Eichert, R. Mars, A. Mitchell and M. Rushworth for their excellent discussion. This study was supported by the Wellcome Trust (grant no. WT091681MA to T.D.G., grant no. WT092606AIA to C.I.P. and grant no. WT110198 to B.W.); the Max Planck Society (to A.D.F. and A.A.); the European Research Council (MECHIDENT to C.I.P.); and the National Institutes of Health (Matthew Howard III with T.D.G. and C.I.P., grant no. R01-DC04290).
Author information
Authors and Affiliations
Contributions
C.I.P., F.B., A.D.F. and T.D.G. conceived the study. F.B., B.W., G.G., F.D., A.A. and C.I.P. conducted the study and the analyses. F.B., B.W., G.G., F.D., W.H., A.A., A.D.F., T.D.G. and C.I.P. provided the materials. C.I.P., F.B. and B.W. wrote the paper with revisions from coauthors G.G., F.D., W.H., A.A., A.D.F. and T.D.G.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Afonso Silva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Macaque deterministic tractography.
Axial (left) and sagittal (right) slices showing diffusion weighted fractional anisotropy (FA) map overlaid with deterministic tractography. The sagittal slice shows the auditory pathways coursing to inferior frontal cortex via a ventral pathway medially involving the Uncinate Fasciculus/Extreme Capsule (UF/EC) pathway and laterally via the Middle Longitudinal Fasciculus (MdLF). No clear dorsal pathway from auditory cortex is observed using deterministic tractography. Diffusion directions, green: anterior-posterior; blue: dorsal-ventral; red: medial-lateral.
Extended Data Fig. 2 Lateral sulcus exclusion mask in the macaques.
To avoid artefactual dorsal pathway voxels not emanating from auditory cortex, an exclusion mask covering the full extent of the Sylvian fissure (lateral sulcus) separating the superior temporal plane and dorsal regions in frontal and parietal cortex was defined. Omission of this mask causes strong false positive dorsal pathways involvement because some auditory cortex voxels in the superior temporal plane are mislocalized to the dorsal lateral sulcus owing to partial volume effects. The lateral sulcus exclusion mask ensured that the analyses are more conservative with regards to spurious dorsal supra-temporal plane to inferior frontal cortex pathway connectivity.
Extended Data Fig. 3 Example comparison of probabilistic dMRI to a macaque fibre pathways atlas coronal section.
Comparison of one of the coronal slices (left) showing tractography from the MM seed region, with a similarly located coronal section (right) from the Schmahmann and Pandya macaque brain pathways atlas13 (copyright permission obtained from Oxford Publishing Limited), suggests that the dorsal pathways that we observed involve the macaque AF and parts of the Superior Longitudinal Fasciculus (SLF). SLF II/III: Superior Longitudinal Fasciculus II/III; AF: Arcuate Fasciculus; EC: Extreme Capsule pathway (Uncinate Fasciculus is visible on more anterior coronal sections); MdLF: Middle Longitudinal Fasciculus.
Supplementary information
Supplementary Information
Supplementary Information.
Rights and permissions
About this article
Cite this article
Balezeau, F., Wilson, B., Gallardo, G. et al. Primate auditory prototype in the evolution of the arcuate fasciculus. Nat Neurosci 23, 611–614 (2020). https://doi.org/10.1038/s41593-020-0623-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0623-9
This article is cited by
-
Language evolution and computational capabilities: conceptualization of the first language units
International Journal of Anthropology and Ethnology (2023)
-
The relevance of the unique anatomy of the human prefrontal operculum to the emergence of speech
Communications Biology (2023)
-
Chimpanzees produce diverse vocal sequences with ordered and recombinatorial properties
Communications Biology (2022)
-
The evolution of hierarchical structure building capacity for language and music: a bottom-up perspective
Primates (2022)
-
Semiotics and the Origin of Language in the Lower Palaeolithic
Journal of Archaeological Method and Theory (2021)