Abstract
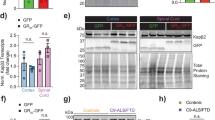
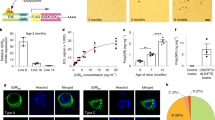
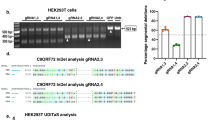
Hexanucleotide expansions in C9orf72, which encodes a predicted guanine exchange factor, are the most frequent genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Although repeat expansion has been established to generate toxic products, mRNAs encoding the C9ORF72 protein are also reduced in affected individuals. In this study, we tested how C9ORF72 protein levels affected repeat-mediated toxicity. In somatic transgenic mice expressing 66 GGGGCC repeats, inactivation of one or both endogenous C9orf72 alleles provoked or accelerated, respectively, early death. In mice expressing a C9orf72 transgene with 450 repeats that did not encode the C9ORF72 protein, inactivation of one or both endogenous C9orf72 alleles exacerbated cognitive deficits, hippocampal neuron loss, glial activation and accumulation of dipeptide-repeat proteins from translation of repeat-containing RNAs. Reduced C9ORF72 was shown to suppress repeat-mediated elevation in autophagy. These efforts support a disease mechanism in ALS/FTD resulting from reduced C9ORF72, which can lead to autophagy deficits, synergizing with repeat-dependent gain of toxicity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
Levine, T. P., Daniels, R. D., Gatta, A. T., Wong, L. H. & Hayes, M. J. The product of C9orf72, a gene strongly implicated in neurodegeneration, is structurally related to DENN Rab-GEFs. Bioinformatics 29, 499–503 (2013).
Zu, T. et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc. Natl Acad. Sci. USA 108, 260–265 (2011).
Zu, T. et al. RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl Acad. Sci. USA 110, E4968–E4977 (2013).
Mori, K. et al. Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol. 126, 881–893 (2013).
Ash, P. E. et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77, 639–646 (2013).
Balendra, R. & Isaacs, A. M. C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat. Rev. Neurol. 14, 544–558 (2018).
Mackenzie, I. R. et al. Quantitative analysis and clinico-pathological correlations of different dipeptide repeat protein pathologies in C9ORF72 mutation carriers. Acta Neuropathol. 130, 845–861 (2015).
Li, N. & Lagier-Tourenne, C. Nuclear pores: the gate to neurodegeneration. Nat. Neurosci. 21, 156–158 (2018).
Saberi, S. et al. Sense-encoded poly-GR dipeptide repeat proteins correlate to neurodegeneration and uniquely co-localize with TDP-43 in dendrites of repeat-expanded C9orf72 amyotrophic lateral sclerosis. Acta Neuropathol. 135, 459–474 (2018).
Mackenzie, I. R. et al. Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations. Acta Neuropathol. 126, 859–879 (2013).
Lagier-Tourenne, C. et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc. Natl Acad. Sci. USA 110, E4530–E4539 (2013).
Wojciechowska, M. & Krzyzosiak, W. J. Cellular toxicity of expanded RNA repeats: focus on RNA foci. Hum. Mol. Genet. 20, 3811–3821 (2011).
Haeusler, A. R., Donnelly, C. J. & Rothstein, J. D. The expanding biology of the C9orf72 nucleotide repeat expansion in neurodegenerative disease. Nat. Rev. Neurosci. 17, 383–395 (2016).
Burguete, A. S. et al. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. eLife 4, e08881 (2015).
Jiang, J. et al. Gain of toxicity from ALS/FTD-linked repeat expansions in C9ORF72 is alleviated by antisense oligonucleotides targeting GGGGCC-containing RNAs. Neuron 90, 535–550 (2016).
Liu, Y. et al. C9orf72 BAC mouse model with motor deficits and neurodegenerative features of ALS/FTD. Neuron 90, 521–534 (2016).
Chew, J. et al. Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science 348, 1151–1154 (2015).
Belzil, V. V. et al. Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol. 126, 895–905 (2013).
Donnelly, C. J. et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80, 415–428 (2013).
Frick, P. et al. Novel antibodies reveal presynaptic localization of C9orf72 protein and reduced protein levels in C9orf72 mutation carriers. Acta Neuropathol. Commun. 6, 72 (2018).
Waite, A. J. et al. Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol. Aging 35, 1779 e1775–1779 e1713 (2014).
Xi, Z. et al. Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am. J. Hum. Genet. 92, 981–989 (2013).
Xi, Z. et al. The C9orf72 repeat expansion itself is methylated in ALS and FTLD patients. Acta Neuropathol. 129, 715–727 (2015).
Ciura, S. et al. Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann. Neurol. 74, 180–187 (2013).
Therrien, M., Rouleau, G. A., Dion, P. A. & Parker, J. A. Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLoS ONE 8, e83450 (2013).
O’Rourke, J. G. et al. C9orf72 is required for proper macrophage and microglial function in mice. Science 351, 1324–1329 (2016).
Atanasio, A. et al. C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci. Rep. 6, 23204 (2016).
Ugolino, J. et al. Loss of C9orf72 enhances autophagic activity via deregulated mTOR and TFEB signaling. PLoS Genet. 12, e1006443 (2016).
Burberry, A. et al. Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci. Transl. Med. 8, 347ra393 (2016).
Sullivan, P. M. et al. The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta Neuropathol. Commun. 4, 51 (2016).
Koppers, M. et al. C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann. Neurol. 78, 426–438 (2015).
Shi, Y. et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 24, 313–325 (2018).
Sellier, C. et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 35, 1276–1297 (2016).
Yang, M. et al. A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci. Adv. 2, e1601167 (2016).
Webster, C. P. et al. The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J. 35, 1656–1676 (2016).
Zhang, Y. et al. The C9orf72-interacting protein Smcr8 is a negative regulator of autoimmunity and lysosomal exocytosis. Genes Dev. 32, 929–943 (2018).
Ho, W. Y. et al. The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy. Autophagy 15, 827–842 (2019).
Liu, E. Y. et al. C9orf72 hypermethylation protects against repeat expansion-associated pathology in ALS/FTD. Acta Neuropathol. 128, 525–541 (2014).
Zhang, D., Iyer, L. M., He, F. & Aravind, L. Discovery of novel DENN proteins: implications for the evolution of eukaryotic intracellular membrane structures and human disease. Front. Genet. 3, 283 (2012).
Scholz, J., Niibori, Y., W Frankland, P. & P Lerch, J. Rotarod training in mice is associated with changes in brain structure observable with multimodal MRI. Neuroimage 107, 182–189 (2015).
Nixon, R. A., Yang, D. S. & Lee, J. H. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy 4, 590–599 (2008).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222 (2016).
Ciechanover, A. The ubiquitin-proteasome proteolytic pathway. Cell 79, 13–21 (1994).
Chitiprolu, M. et al. A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy. Nat. Commun. 9, 2794 (2018).
Jiang, J. & Ravits, J. Pathogenic mechanisms and therapy development for C9orf72 amyotrophic lateral sclerosis/frontotemporal dementia. Neurotherapeutics 16, 1115–1132 (2019).
Nassif, M., Woehlbier, U. & Manque, P. A. The enigmatic role of C9ORF72 in autophagy. Front. Neurosci. 11, 442 (2017).
Taylor, J. P., Brown, R. H. Jr. & Cleveland, D. W. Decoding ALS: from genes to mechanism. Nature 539, 197–206 (2016).
Roy Sarkar, S. & Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 328, 98–104 (2019).
Kramer, N. J. et al. Spt4 selectively regulates the expression of C9orf72 sense and antisense mutant transcripts. Science 353, 708–712 (2016).
Su, Z. et al. Discovery of a biomarker and lead small molecules to target r(GGGGCC)-associated defects in c9FTD/ALS. Neuron 83, 1043–1050 (2014).
Gendron, T. F. et al. Cerebellar c9RAN proteins associate with clinical and neuropathological characteristics of C9ORF72 repeat expansion carriers. Acta Neuropathol. 130, 559–573 (2015).
Parone, P. A. et al. Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis. J. Neurosci. 33, 4657–4671 (2013).
Shao, C. et al. Mitotic recombination produces the majority of recessive fibroblast variants in heterozygous mice. Proc. Natl Acad. Sci. USA 96, 9230–9235 (1999).
Jiang, L. L. et al. Membralin deficiency dysregulates astrocytic glutamate homeostasis leading to ALS-like impairment. J. Clin. Invest. 129, 3103–3120 (2019).
Acknowledgements
We thank B. Myers, M. Maldonado, J. Kim, J. Lim, J. Yasis, C.J. Heyser, D. Ditsworth, K. Osborn and J. Chew for their advice and technical assistance. We thank M. Fugere and B. Kaspar at AveXis for providing help in sorting mouse ESC-derived motor neurons. We thank Ionis Pharmaceuticals for providing ASOs. We thank all members of the D.W.C., C.L.-T., J.R., and S.D.C. groups for critical suggestions on this project. This work was supported by grants from NINDS/NIH R01-NS27036 to D.W.C. and S.D.C. and R01-NS087227 to C.L.-T.; from the NIA/NIH-supported UCSD Alzheimer’s Disease Research Center (P50-AG005131) to C.L.-T. and D.W.C.; from Target ALS to C.L.-T. and J.R.; from NINDS/NIH R35-NS097273, P01-NS084974 and R01-NS088689 to L.P.; from P01-NS099114 to T.G. and L.P.; and from Target ALS to T.G., L.P. and Y.Z. C.L.-T. is the recipient of the Healey Family ALS Endowed Chair for Research. Q.Z. was the recipient of a Milton Safenowitz Postdoctoral Fellowship and a STARTER grant from the ALS Association. J.J. was the recipient of a career development grant from the Muscular Dystrophy Association (479769).
Author information
Authors and Affiliations
Contributions
Q.Z., J.J., S.D.C., C.L.-T. and D.W.C. designed the research. Q.Z., J.J., T.F.G., A.R.L.S., H.X., L.P., J.R., S.D.C., C.L.-T. and D.W.C. analyzed the data. Q.Z., J.J., T.F.G., M.M.D., L.J., A.T., S.D.G., S.G.D., M.J.R., P.K. and Y.Z. performed research. Q.Z., J.J., S.D.C., C.L.-T. and D.W.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
D.W.C. is a consultant for Ionis Pharmaceuticals. The other authors report no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8.
Source data
Source Data Fig. 1
Unmodified gels for Fig. 2
Source Data Fig. 2
Unmodified gels for Fig. 5
Rights and permissions
About this article
Cite this article
Zhu, Q., Jiang, J., Gendron, T.F. et al. Reduced C9ORF72 function exacerbates gain of toxicity from ALS/FTD-causing repeat expansion in C9orf72. Nat Neurosci 23, 615–624 (2020). https://doi.org/10.1038/s41593-020-0619-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0619-5
This article is cited by
-
Artificial microRNA suppresses C9ORF72 variants and decreases toxic dipeptide repeat proteins in vivo
Gene Therapy (2024)
-
Translating the ALS Genetic Revolution into Therapies: A Review
Current Treatment Options in Neurology (2024)
-
Pathological insights from amyotrophic lateral sclerosis animal models: comparisons, limitations, and challenges
Translational Neurodegeneration (2023)
-
A toxic gain-of-function mechanism in C9orf72 ALS impairs the autophagy-lysosome pathway in neurons
Acta Neuropathologica Communications (2023)
-
C9orf72-catalyzed GTP loading of Rab39A enables HOPS-mediated membrane tethering and fusion in mammalian autophagy
Nature Communications (2023)