Abstract
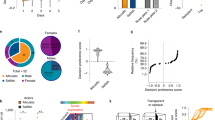
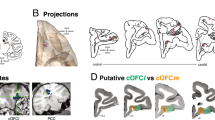
Social behaviors recruit multiple cognitive operations that require interactions between cortical and subcortical brain regions. Interareal synchrony may facilitate such interactions between cortical and subcortical neural populations. However, it remains unknown how neurons from different nodes in the social brain network interact during social decision-making. Here we investigated oscillatory neuronal interactions between the basolateral amygdala and the rostral anterior cingulate gyrus of the medial prefrontal cortex while monkeys expressed context-dependent positive or negative other-regarding preference (ORP), whereby decisions affected the reward received by another monkey. Synchronization between the two nodes was enhanced for a positive ORP but suppressed for a negative ORP. These interactions occurred in beta and gamma frequency bands depending on the area contributing the spikes, exhibited a specific directionality of information flow associated with a positive ORP and could be used to decode social decisions. These findings suggest that specialized coordination in the medial prefrontal–amygdala network underlies social-decision preferences.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Behavioral and neural data presented in this paper are available at https://github.com/changlabneuro/medial-prefrontal-amygdala-coordination-analyses.
Code availability
Behavioral and neural data analysis codes central to this paper are available at https://github.com/changlabneuro/medial-prefrontal-amygdala-coordination-analyses.
References
Behrens, T. E. J., Hunt, L. T. & Rushworth, M. F. S. The computation of social behavior. Science 324, 1160–1164 (2009).
Bhanji, J. P. & Delgado, M. R. The social brain and reward: social information processing in the human striatum. Wiley Interdiscip. Rev. Cogn. Sci. 5, 61–73 (2014).
Sliwa, J. & Freiwald, W. A. A dedicated network for social interaction processing in the primate brain. Science 356, 745–749 (2017).
Ruff, C. C. & Fehr, E. The neurobiology of rewards and values in social decision making. Nat. Rev. Neurosci. 15, 549–562 (2014).
Seo, H. & Lee, D. Neural basis of learning and preference during social decision-making. Curr. Opin. Neurobiol. 22, 990–995 (2012).
Chang, S. W. C., Gariépy, J.-F. & Platt, M. L. Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci. 16, 243–250 (2013).
Haroush, K. & Williams, Z. M. Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 160, 1233–1245 (2015).
Noritake, A., Ninomiya, T. & Isoda, M. Social reward monitoring and valuation in the macaque brain. Nat. Neurosci. 21, 1452–1462 (2018).
Chang, S. W. C. et al. Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl Acad. Sci. USA 112, 16012–16017 (2015).
Grabenhorst, F., Báez-Mendoza, R., Genest, W., Deco, G. & Schultz, W. Primate amygdala neurons simulate decision processes of social partners. Cell 177, 986–998.e15 (2019).
Munuera, J., Rigotti, M. & Salzman, C. D. Shared neural coding for social hierarchy and reward value in primate amygdala. Nat. Neurosci. 21, 415–423 (2018).
Azzi, J. C. B., Sirigu, A. & Duhamel, J.-R. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc. Natl Acad. Sci. USA 109, 2126–2131 (2012).
Baez-Mendoza, R., Harris, C. J. & Schultz, W. Activity of striatal neurons reflects social action and own reward. Proc. Natl Acad. Sci. USA 110, 16634–16639 (2013).
Falcone, R., Brunamonti, E., Ferraina, S. & Genovesio, A. Neural encoding of self and another agent’s goal in the primate prefrontal cortex: human–monkey interactions. Cereb. Cortex 26, 4613–4622 (2016).
Nummela, S. U., Jovanovic, V., Mothe, Ldela & Miller, C. T. Social context-dependent activity in marmoset frontal cortex populations during natural conversations. J. Neurosci. 37, 7036–7047 (2017).
Apps, M. A. J., Rushworth, M. F. S. & Chang, S. W. C. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 90, 692–707 (2016).
Hill, M. R., Boorman, E. D. & Fried, I. Observational learning computations in neurons of the human anterior cingulate cortex. Nat. Commun. 7, 12722 (2016).
Zaki, J. & Ochsner, K. The neuroscience of empathy: progress, pitfalls and promise. Nat. Neurosci. 15, 675–680 (2012).
Mars, R. B. et al. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 6, 189 (2012).
Amadei, E. A. et al. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297–301 (2017).
Allsop, S. A. et al. Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342.e18 (2018).
Zhan, Y. et al. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Carmichael, S. T. & Price, J. L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615–641 (1995).
Klavir, O., Genud-Gabai, R. & Paz, R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron 80, 1290–1300 (2013).
Pesaran, B. et al. Investigating large-scale brain dynamics using field potential recordings: analysis and interpretation. Nat. Neurosci. 21, 903–919 (2018).
Fries, P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480 (2005).
Chang, S. W. C., Winecoff, A. A. & Platt, M. L. Vicarious reinforcement in rhesus macaques (Macaca mulatta). Front. Neurosci. 5, 27 (2011).
Chang, S. W. C., Barter, J. W., Ebitz, R. B., Watson, K. K. & Platt, M. L. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl Acad. Sci. USA 109, 959–964 (2012).
Paxinos, G., Huang, X.-F. & Toga, A. W. The Rhesus Monkey Brain in Stereotaxic Coordinates (Academic Press, 1999).
Baccalá, L. A. & Sameshima, K. Partial directed coherence: a new concept in neural structure determination. Biol. Cybern. 84, 463–474 (2001).
Buzsáki, G. & Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 35, 203–225 (2012).
Hipp, J. F., Engel, A. K. & Siegel, M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron 69, 387–396 (2011).
Womelsdorf, T., Fries, P., Mitra, P. P. & Desimone, R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439, 733–736 (2006).
Wong, Y. T., Fabiszak, M. M., Novikov, Y., Daw, N. D. & Pesaran, B. Coherent neuronal ensembles are rapidly recruited when making a look-reach decision. Nat. Neurosci. 19, 327–334 (2016).
Kahana, M. J., Sekuler, R., Caplan, J. B., Kirschen, M. & Madsen, J. R. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784 (1999).
Fujisawa, S. & Buzsáki, G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 72, 153–165 (2011).
Adhikari, A., Topiwala, M. A. & Gordon, J. A. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65, 257–269 (2010).
Antzoulatos, E. G. & Miller, E. K. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron 83, 216–225 (2014).
Brincat, S. L. & Miller, E. K. Frequency-specific hippocampal–prefrontal interactions during associative learning. Nat. Neurosci. 18, 576–581 (2015).
Taub, A. H., Perets, R., Kahana, E. & Paz, R. Oscillations synchronize amygdala-to-prefrontal primate circuits during aversive learning. Neuron 97, 291–298.e3 (2018).
Buschman, T. J. & Miller, E. K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1862 (2007).
Engel, A. K., Fries, P. & Singer, W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716 (2001).
Engel, A. K. & Fries, P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165 (2010).
Spitzer, B. & Haegens, S. Beyond the status quo: a role for beta oscillations in endogenous content (re)activation. eNeuro 4, ENEURO.0170-17.2017 (2017).
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Jia, X. & Kohn, A. Gamma rhythms in the brain. PLoS Biol. 9, e1001045 (2011).
Livneh, U., Resnik, J., Shohat, Y. & Paz, R. Self-monitoring of social facial expressions in the primate amygdala and cingulate cortex. Proc. Natl Acad. Sci. USA 109, 18956–18961 (2012).
Gothard, K. M., Battaglia, F. P., Erickson, C. A., Spitler, K. M. & Amaral, D. G. Neural responses to facial expression and face identity in the monkey amygdala. J. Neurophysiol. 97, 1671–1683 (2007).
Grabenhorst, F., Hernádi, I. & Schultz, W. Prediction of economic choice by primate amygdala neurons. Proc. Natl Acad. Sci. USA 109, 18950–18955 (2012).
Sutton, R. S. & Barto, A. G. Reinforcement Learning: An Introduction (A Bradford Book, 1998).
Liu, Y., Yttri, E. A. & Snyder, L. H. Intention and attention: different functional roles for LIPd and LIPv. Nat. Neurosci. 13, 495–500 (2010).
Chung, J. E. et al. A fully automated approach to spike sorting. Neuron 95, 1381–1394.e6 (2017).
Bokil, H., Andrews, P., Kulkarni, J. E., Mehta, S. & Mitra, P. P. Chronux: a platform for analyzing neural signals. J. Neurosci. Methods 192, 146–151 (2010).
Bastos, A. M. & Schoffelen, J.-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 9, 175 (2015).
Jarvis, M. R. & Mitra, P. P. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 13, 717–749 (2001).
Omidvarnia, A. H. et al. Measuring time-varying information flow in scalp EEG signals: orthogonalized partial directed coherence. IEEE Trans. Biomed. Eng. 61, 680–693 (2013).
Saez, A., Rigotti, M., Ostojic, S., Fusi, S. & Salzman, C. D. Abstract context representations in primate amygdala and prefrontal cortex. Neuron 87, 869–881 (2015).
Acknowledgements
We are extremely grateful to B. Pesaran for his guidance on examining oscillatory neural processes throughout the duration of this research. We especially thank D. Lee and A. Kwan for their thoughtful discussions and suggestions on improving this work. We also thank A. Nair and S. Fan for insightful comments on the manuscript. This work was supported by the National Institute of Mental Health (R01MH110750, R01MH120081, R21MH107853 and R00MH099093), the Alfred P. Sloan Foundation (FG-2015-66028) and the Teresa Seessel Postdoctoral Fellowship.
Author information
Authors and Affiliations
Contributions
S.W.C.C. and O.D.M. designed the study and wrote the paper. O.D.M. performed the experiments. C.C.J.C., N.A.F., O.D.M. and S.W.C.C. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Ziv Williams and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11.
Supplementary Table
Supplementary Table 1. Contains the P values for all the occasions where we use P < 0.05 criterion in the figures (main and supplementary figures) to indicate significant symbols and calculations.
Rights and permissions
About this article
Cite this article
Dal Monte, O., Chu, C.C.J., Fagan, N.A. et al. Specialized medial prefrontal–amygdala coordination in other-regarding decision preference. Nat Neurosci 23, 565–574 (2020). https://doi.org/10.1038/s41593-020-0593-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0593-y
This article is cited by
-
Chemogenetic dissection of a prefrontal-hypothalamic circuit for socially subjective reward valuation in macaques
Nature Communications (2023)
-
Frontal neurons driving competitive behaviour and ecology of social groups
Nature (2022)
-
Reciprocal cortico-amygdala connections regulate prosocial and selfish choices in mice
Nature Neuroscience (2022)
-
Prefrontal cortex interactions with the amygdala in primates
Neuropsychopharmacology (2022)
-
Neural implementation of computational mechanisms underlying the continuous trade-off between cooperation and competition
Nature Communications (2022)