Abstract
In humans and rodents, the perception of control during stressful events has lasting behavioral consequences. These consequences are apparent even in situations that are distinct from the stress context, but how the brain links prior stressful experience to subsequent behaviors remains poorly understood. By assessing innate defensive behavior in a looming-shadow task, we show that the initiation of an escape response is preceded by an increase in the activity of corticotropin-releasing hormone (CRH) neurons in the paraventricular nucleus (PVN) of the hypothalamus (CRHPVN neurons). This anticipatory increase is sensitive to stressful stimuli that have high or low levels of outcome control. Specifically, experimental stress with high outcome control increases CRHPVN neuron anticipatory activity, which increases escape behavior in an unrelated context. By contrast, stress with no outcome control prevents the emergence of this anticipatory activity and decreases subsequent escape behavior. These observations indicate that CRHPVN neurons encode stress controllability and contribute to shifts between active and passive innate defensive strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All relevant data and analysis tools are available upon reasonable request from the authors.
Code availability
Scripts used to analyze fiber photometry and detect miniscope events are deposited at https://github.com/leomol/FPA and https://github.com/leomol/MSA.
References
Kushner, M. G., Riggs, D. S., Foa, E. B. & Miller, S. M. Perceived controllability and the development of posttraumatic stress disorder (PTSD) in crime victims. Behav. Res. Ther. 31, 105–110 (1993).
Hartley, C. A., Gorun, A., Reddan, M. C., Ramirez, F. & Phelps, E. A. Stressor controllability modulates fear extinction in humans. Neurobiol. Learn. Mem. 113, 149–156 (2014).
Maier, S. F. & Watkins, L. R. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 29, 829–841 (2005).
Maier, S. F. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol. Stress 1, 12–22 (2014).
Boeke, E. A., Moscarello, J. M., LeDoux, J. E., Phelps, E. A. & Hartley, C. A. Active avoidance: neural mechanisms and attenuation of Pavlovian conditioned responding. J. Neurosci. 37, 4808–4818 (2007).
Füzesi, T., Daviu, N., Wamsteeker Cusulin, J. I., Bonin, R. P. & Bains, J. S. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat. Commun. 7, 11937 (2016).
Zhang, R. et al. Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry 22, 733–744 (2017).
Ramot, A. et al. Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat. Neurosci. 20, 385–388 (2017).
Sterley, T.-L. et al. Social transmission and buffering of synaptic changes after stress. Nat. Neurosci. 21, 393–403 (2018).
De Marco, R. J., Thiemann, T., Groneberg, A. H., Herget, U. & Ryu, S. Optogenetically enhanced pituitary corticotroph cell activity post-stress onset causes rapid organizing effects on behaviour. Nat. Commun. 7, 2620 (2016).
Kim, J. et al. Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci. 22, 576–585 (2019).
Maier, S. F., Ryan, S. M., Barksdale, C. M. & Kalin, N. H. Stressor controllability and the pituitary–adrenal system. Behav. Neurosci. 100, 669–674 (1986).
Liu, X., Tang, X. & Sanford, L. D. Stressor controllability and Fos expression in stress regulatory regions in mice. Physiol. Behav. 97, 321–326 (2009).
Yilmaz, M. & Meister, M. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. 23, 2011–2015 (2013).
Chow, B. Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Cui, G. et al. Deep brain optical measurements of cell type-specific neural activity in behaving mice. Nat. Protoc. 9, 1213–1228 (2014).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Fadok, J. P. et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).
Baratta, M. V. et al. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience 8, 1495–1503 (2007).
Kuzmiski, J. B., Marty, V., Baimoukhametova, D. V. & Bains, J. S. Stress-induced priming of glutamate synapses unmasks associative short-term plasticity. Nat. Neurosci. 13, 1257–1264 (2010).
Fulcher, B. D. & Jones, N. S. hctsa: a computational framework for automated time-series phenotyping using massive feature extraction. Cell Syst. 5, 527–531.e3 (2017).
Blanchard, R. J., Flannelly, K. J. & Blanchard, D. C. Defensive behaviors of laboratory and wild Rattus norvegicus. J. Comp. Psychol. 100, 101–107 (1986).
Shang, C. et al. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 348, 1472–1477 (2015).
Evans, D. A. et al. A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594 (2018).
Hu, Y. et al. A translational study on looming-evoked defensive response and the underlying subcortical pathway in autism. Sci. Rep. 7, 14755 (2017).
Huang, L. et al. A retinoraphe projection regulates serotonergic activity and looming-evoked defensive behaviour. Nat. Commun. 8, 14908 (2017).
Wang, L., Chen, I. Z. & Lin, D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85, 1344–1358 (2015).
Youngstrom, T. G., Weiss, M. L. & Nunez, A. A. Retinofugal projections to the hypothalamus, anterior thalamus and basal forebrain in hamsters. Brain Res. Bull. 26, 403–411 (1991).
Schaechter, J. D. & Sadun, A. A. A second hypothalamic nucleus receiving retinal input in man: the paraventricular nucleus. Brain Res. 340, 243–250 (1985).
Porter, J. P. & Brody, M. J. Neural projections from paraventricular nucleus that subserve vasomotor functions. Am. J. Physiol. 248, R271–R281 (1985).
Feldman Barrett, L. & Finlay, B. L. Concepts, goals and the control of survival-related behaviors. Curr. Opin. Behav. Sci. 24, 172–179 (2018).
Helmreich et al. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 11, 121–128 (2001).
Lucas, M. et al. Long-term effects of controllability or the lack of it on coping abilities and stress resilience in the rat. Stress 17, 423–430 (2014).
Maier, S. F. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J. Exp. Psychol. Anim. Behav. Process 16, 137–149 (1990).
Amat, J. et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 8, 365–371 (2005).
Gross, C. T. & Canteras, N. S. The many paths to fear. Nat. Rev. Neurosci. 13, 651–658 (2012).
Corcoran, K. A. & Quirk, G. J. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J. Neurosci. 27, 840–844 (2012).
Nicholson, A. A. et al. Dynamic causal modeling in PTSD and its dissociative subtype: bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum. Brain Mapp. 38, 5551–5561 (2017).
Qi, S. et al. How cognitive and reactive fear circuits optimize escape decisions in humans. Proc. Natl Acad. Sci. USA 115, 3186–3191 (2018).
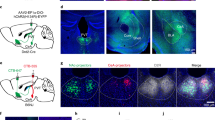
Cusulin, J. I. W., Füzesi, T., Watts, A. G. & Bains, J. S. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-cre mutant mice. PLoS ONE 8, e64943 (2013).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Guo, Q. et al. Multi-channel fiber photometry for population neuronal activity recording. Biomed. Opt. Express 6, 3919–3931 (2015).
MATLAB and Classification Learner App v.R2018b (The MathWorks Inc., 2018).
Acknowledgements
We thank the expert technical support of C. Breiteneder, M. Tsutsui, C. Martinez and L. A. Molina. We are grateful for the support of the Cumming School of Medicine Optogenetics Core Facility. This work was supported by an operating grant to J.S.B. from the Canadian Institutes for Health Research (FDN-148440) and the Brain Canada Neurophotonics Platform. N.D. and T.-L.S. are supported by Fellowships from Alberta Innovates-Health Solutions.
Author information
Authors and Affiliations
Contributions
N.D. designed and conducted experiments, analyzed data, prepared figures and wrote the manuscript. T.F. designed and conducted behavioral experiments, analyzed data and prepared figures. D.G.R. developed video and photometry analysis tools, analyzed behavioral data and reviewed the manuscript. N.P.R. and T.-L.S. conducted slice electrophysiology experiments and analyzed data. G.P. developed the accelerometer software and analysis, the artificial decoder and software for automating escape analysis. J.S.B. developed and supervised the project, analyzed data, constructed figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Lieselot Carrette, Olivier George, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
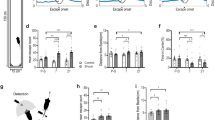
Extended Data Fig. 1 Prior experience changes defensive strategy.
a, Protocol for the second exposure to looming-shadow test. b, Behavioral analysis showing individual trials (trials/mice, n = 55, N = 11) and c, summary of all trials for the 3 behavioral outputs analyzed. d, Representation of the starting point of subject in each trial for the second exposure to the looming-shadow test. e, Data compiled and presented as fraction of trials showing a given behavior in a mouse. Escape (N = 11, Paired t-test, two tailed t(10) = 2.472, p = 0.0330, 95% CI: 1.973 to 38.03), freeze (Wilcox test, two-tailed, w = −3.000, p = 0.750), and no response (Paired t-test, two-tailed, t(10) = 3.194, p = 0.0096, 95% CI: −30.86 to −5.50). f, Representation of the starting point of subject in each trial for the photoinhibition experiment (left CRHeYFP, right CRHArch3.0). Data shown are means ± s.e.m.
Extended Data Fig. 2 Relationship between GCaMP signal and visual stimulus assessed using different parameters.
a, (i) Individual traces (gray) and mean (overlaid black) of CRHPVN activity in response to an object that tracks in a horizontal plane across the sky but does not advance (trials/mice, n = 9, N = 3). The length of the stimulus was 8 s and it was moving at a constant speed of 6 cm/s. (ii) Individual traces (gray) and mean (overlaid black) of CRHPVN activity in response to an object that remains static in a horizontal plane across the sky for the duration of the experiment (trials/mice, n = 15, N = 3) b, Individual traces (gray) and mean (overlaid green) of CRHPVN activity in response to shadow presentation corresponding to trials resulting in a freezing behavior for Naïve mice (trials/mice, n = 4, N = 3). c, Comparison between manual vs automated assessment of escape onset in the looming-shadow test (Paired t-test, two tailed, t(20) = 1.027, p = 0.3169, 95% CI −0.7580 to 0.2580). d, Average z-score of CRHPVN calcium response with individual trials time-locked to the onset of the escape when assessed manually using a frame by frame approach. Escape response indicated by dashed line and red arrow (trials/mice n = 21, N = 5). Solid lines represent average, and the shaded areas indicate SEM. Data shown are means ± s.e.m.
Extended Data Fig. 3 Avoidance behavior during controllable stress training.
Avoidance behavior in controllable group (N = 10, day 1 to day 3, repeated measure ANOVA, F(2,18) = 10.17, p = 0.0011, Bonferroni’s multiple comparisons test, Day1 vs day 2 p > 0.999, 95% CI −17.00 to 10.00. Day 2 vs day 3 p = 0.0074, 95% CI −35.00 to −8.00. Day 3 vs day 1 p = 0.0016, 95% CI −31.50 to −4.50). Data shown are means ± s.e.m.
Extended Data Fig. 4 Effects of controllability training on basal glutamate transmission and intrinsic excitability of CRHPVN neurons.
a, sEPSC amplitude and frequency for naïve, controllable and uncontrollable stress 24 h after the last training session (cells/mice, Naive = 21/4, Controllable = 16/5, Uncontrollable = 19/5; amplitude, one-way ANOVA, f(2,53) = 0.8868, p = 0.4180, Bonferroni’s multiple comparison, Naïve vs controllable p = 0.7944, 95% CI −2.966 to 7.935. Naïve vs Uncontrollable p = 0.7743, 95% CI −2.796 to 7.936. Controllable vs Uncontrollable p > 0.999, 95% CI −5.497 to 5.654; frequency, one-way ANOVA, F(2,53) = 1.405, p = 0.2544, Bonferroni’s multiple comparison, Naïve vs controllable p = 0.6639, 95% CI −4.640 to 2.188. Naïve vs Uncontrollable p = 0.6509, 95% CI −2.059 to 4.456. Controllable vs Uncontrollable p = 0.2244, 95% CI −5.915 to 1.067). b, Bar graph showing no significant changes in baseline PPR between groups (cells/mice, Controllable = 21/9, Uncontrollable = 25/10, Unpaired t-test, two-tailed, t(44) = 1.037, p = 0.3053, 95% CI −0.05364 to 0.1674). c, F-I plot shows spike frequency for each depolarizing current step (cells/mice, Naive = 18/4, Controllable = 30/5, Uncontrollable = 27/5 -2way ANOVA current step x group F(18,648) = 0.8444, p = 0.6476). Data shown are means ± s.e.m.
Extended Data Fig. 5 Peak GCaMP responses to footshock following controllable and uncontrollable stress.
a, Individual z-scores of baseline (shock onset) and footshock peak values for controllable (right, blue circles, Day 1 trials/mice = 120/6; Baseline min = −3.369, 25% = −0.1015, med = 1.118, 75% = 2.118, max = 7.730. FS peak min = −1.849, 25% = 4.602, med = 6.639, 75% = 9.195, max = 19.19; Wilcoxon signed rank test, two-tailed, W = 7248, p < 0.0001. Day 2 trials/mice = 114/6; Baseline min = −2.906, 25% = 0.4915, med = 1.593, 75% = 3.993, max = 13.14. FS peak min = −2.906, 25% = 4.155, med = 6.818, 75% = 9.533, max = 25.36; Wilcoxon signed rank test, two-tailed, W = 5430, p < 0.0001. Day 3 trials/mice = 119/6; Baseline min = −4.187, 25% = 0.5293, med = 2.544, 75% = 3.808, max = 14.82. FS peak min = −3.033, 25% = 3.302, med = 6.333, 75% = 9.212, max = 20.61; Wilcoxon signed rank test, W = 6399, p < 0.0001) and uncontrollable (orange circles, Day 1 trials/mice = 120/6; Baseline min = −5.375, 25% = −0.6542, med = 0.4421, 75% = 1.974, max = 12.61. FS peak min = −2.018, 25% = 5.927, med = 7.320, 75% = 9.287, max = 17.91; Wilcoxon signed rank test, two-tailed, W = 7230, p < 0.0001. Day 2 trials/mice = 119/6; Baseline min = −5.068, 25% = 0.6244, med = 0.3252, 75% = 1.568, max = 17.68. FS peak min = −0.218, 25% = 4.628, med = 6.598, 75% = 9.112, max = 26.66; Wilcoxon signed rank test, two-tailed, W = 7009, p < 0.0001. Day 3 trials/mice = 120/6; Baseline min = −3.594, 25% = −0.5115, med = 0.6376, 75% = 2.253, max = 16.58. FS peak min = −0.3636, 25% = 4.288, med = 6.584, 75% = 8.212, max = 33.54;Wilcoxon signed rank test, two-tailed, W = 5430, p = 0.0006) group. b, Individual delta z-scores (footshock - baseline) on day 1 (Controllable trials/mice = 120/6; min = -1.061, 25% = 3.809, med = 5.967, 75% = 8.262, max = 15.79. Uncontrollable trials/mice 120/6. min = -0.667, 25% = 4.222, med = 7.493, 75% = 8.956, max = 15.31; Mann-Whitney test, two tailed, U = 62.7, p = 0.0650), day 2 (Controllable trials/mice = 114/6; min = -1.667, 25% = 1.948, med = 4.855, 75% = 7.101, max = 19.22. Uncontrollable trials/mice = 119/6; min = -0.4561, 25% = 3.558, med = 6.533, 75% = 9.064, max = 24.63; Mann-Whitney test, two tailed, U = 5307, p = 0.0041) and day 3 (Controllable trials/mice = 120/6; min = -0.4106, 25% = 1.844, med = 3.499, 75% = 6.173, max = 17.86. Uncontrollable trials/mice = 120/6; min = -0.3787, 25% = 3.421, med = 5.809, 75% = 7.824, max = 24.80; Mann-Whitney test, two tailed, U = 5014, p < 0.0001). c, Average CRHPVN activity on day 1 for both controllable and uncontrollable groups on trial 3, 5, 15, 18 and 20 (n = 6). Data shown in the violin plots are median, 25% and 75% percentile (black bars).
Extended Data Fig. 6 Circulating corticosterone levels after controllability training.
Corticosterone levels after the last day of training of controllable (N = 10) and uncontrollable (N = 10) stress protocol (Unpaired t-test, two-tailed, t(19) = 1.586, p = 0.1301, 95% CI: -147.9 to 20.65). Data shown are means ± s.e.m.
Extended Data Fig. 7 Non-linear clustering visualization in lower dimensional space.
a, Visualization of the data matrix using non-linear clustering in a lower-dimensional space based on the 2 main features extracted from the calcium traces. From top to bottom: day 1 alone, day 2 alone, day 3 alone, all training days combined. b, Confusion matrices showing the classification accuracy (ratio of correct predictions to total predictions made) of the trained decoder from (top to bottom) day 1 to day 3.
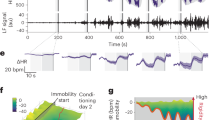
Extended Data Fig. 8 Immobility in response to controllability training.
Immobility during: (a) baseline period (5 min before the tone presentation, unpaired t-test, two-tailed, t(10) = 1.185, p = 0.2636, 95% CI -6.161 to 20.16), (b) during tone presentation, 8 s stimulus presentation (two-way ANOVA, tone x group interaction f(4,40) = 0.5399, p = 0.7073) and (c) between tones (two-way ANOVA, tone x group interaction f(3,30) = 1.823, p = 0.1642). Inter-tone-interval (ITI) = 52 s, (N = 6 each group).Data shown are means ± s.e.m.
Extended Data Fig. 9 Additional photometry data analysis for looming shadow tests following controllability training.
a, Non-responders in uncontrollable group showing individual traces (gray) and average z-score (black) of CRHPVN calcium response during visual stimulus presentation (trials/mice = 4/3). b, Comparison between manual (frame by frame), and automated analysis of the escape reaction time (n = 26, ERT, Paired t-test, two-tailed, t(25) = 0.4067, p = 0.6877, 95% CI -0.5929 to 0.8847). c, Average z-score of CRHPVN calcium response with individual trials time-locked to the onset of the escape. Escape response indicated by dashed line and red arrow (trials/mice n = 33, N = 9). d, Individual z-score values at the baseline (white circles) and at flight response initiation (blue circles, trials/mice n = 33, N = 9; two-tailed paired t-test, t(32) = 4.025, p = 0.0003, 95% CI 0.8558 to 2.610). Solid lines represent average, and the shaded areas indicate s.e.m. Data shown are means ± s.e.m.
Extended Data Fig. 10 Compiled GCaMP data across experiments from all escape trials and all freeze trials.
a, Average z-score of CRHPVN calcium response with all individual trials time-locked to the onset of stimulus. Left panel shows all trials (naïve, controllable and uncontrollable stress; n = 55 trials) that showed an escape response; middle panel shows all trials that showed a freezing response; n = 16 trials). Right panel shows the overlaid responses from escapers and freezers during the shadow presentation. b, Cumulative distributions of z-scores at the end of shadow expansion (5 s from stimulus onset). c, z scores from escape (n = 55) and freeze (n = 16) trails at the 5 s mark of shadow presentation (two-tailed unpaired t-test, Welch’s correction; t(57.55) = 4.184, p < 0.0001, 95% CI 2.266 to 6.426). Data shown are means ± s.e.m.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Rights and permissions
About this article
Cite this article
Daviu, N., Füzesi, T., Rosenegger, D.G. et al. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat Neurosci 23, 398–410 (2020). https://doi.org/10.1038/s41593-020-0591-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0591-0
This article is cited by
-
Fasting-activated ventrolateral medulla neurons regulate T cell homing and suppress autoimmune disease in mice
Nature Neuroscience (2024)
-
Butterflies in the gut: the interplay between intestinal microbiota and stress
Journal of Biomedical Science (2023)
-
Defensive responses: behaviour, the brain and the body
Nature Reviews Neuroscience (2023)
-
Neural circuitry for maternal oxytocin release induced by infant cries
Nature (2023)
-
Systematic evaluation of a predator stress model of depression in mice using a hierarchical 3D-motion learning framework
Translational Psychiatry (2023)