Abstract
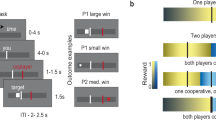
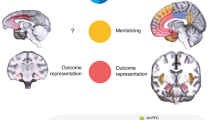
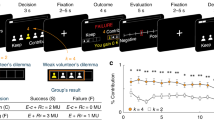
We recorded neural activity in male monkeys playing a variant of the game ‘chicken’ in which they made decisions to cooperate or not cooperate to obtain rewards of different sizes. Neurons in the middle superior temporal sulcus (mSTS)—previously implicated in social perception—signaled strategic information, including payoffs, intentions of the other player, reward outcomes and predictions about the other player. Moreover, a subpopulation of mSTS neurons selectively signaled cooperatively obtained rewards. Neurons in the anterior cingulate gyrus, previously implicated in vicarious reinforcement and empathy, carried less information about strategic variables, especially cooperative reward. Strategic signals were not reducible to perceptual information about the other player or motor contingencies. These findings suggest that the capacity to compute models of other agents has deep roots in the strategic social behavior of primates and that the anterior cingulate gyrus and the mSTS support these computations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data forming the basis of this study are available from the corresponding author upon reasonable request.
Code availability
The custom analysis code used in this study is available from the corresponding author upon reasonable request.
References
Warneken, F. & Tomasello, M. in The Oxford Handbook of Prosocial Behavior (eds Schroeder, D. A. & Graziano, W. G.) Ch. 5 (Oxford Univ. Press, 2015).
Efferson, C. & Fehr, E. Simple moral code supports cooperation. Nature 555, 169–170 (2018).
Decety, J., Bartal, I. B.-A., Uzefovsky, F. & Knafo-Noam, A. Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos. Trans R. Soc. Lond. B Biol. Sci. 371, 20150077 (2016).
Tomlin, D. et al. Agent-specific responses in the cingulate cortex during economic exchanges. Science 312, 1047–1050 (2006).
Rumble, A. C., Van Lange, P. A. M. & Parks, C. D. The benefits of empathy: when empathy may sustain cooperation in social dilemmas. Eur. J. Soc. Psychol. 40, 856–866 (2009).
Batson, C. D. & Moran, T. Empathy-induced altruism in a prisoner’s dilemma. Eur. J. Soc. Psychol. 29, 909–924 (1999).
Saxe, R. & Kanwisher, N. People thinking about thinking people: the role of the temporo–parietal junction in ‘theory of mind’. NeuroImage 19, 1835–1842 (2003).
Chang, S. W. C., Gariépy, J.-F. & Platt, M. L. Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci. 16, 243–250 (2013).
Chang, S. W. C. et al. Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl Acad. Sci. USA 112, 16012–16017 (2015).
Carter, R. M., Bowling, D. L., Reeck, C. & Huettel, S. A. A distinct role of the temporal–parietal junction in predicting socially guided decisions. Science 337, 109–111 (2012).
Yoshida, W., Seymour, B., Friston, K. J. & Dolan, R. J. Neural mechanisms of belief inference during cooperative games. J. Neurosci. 30, 10744–10751 (2010).
Haroush, K. & Williams, Z. M. Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 160, 1233–1245 (2015).
Rushworth, M. F., Mars, R. B. & Sallet, J. Are there specialized circuits for social cognition and are they unique to humans? Curr. Opin. Neurobiol. 23, 436–442 (2013).
Tsao, D. Y., Freiwald, W. A., Knutsen, T. A., Mandeville, J. B. & Tootell, R. B. H. Faces and objects in macaque cerebral cortex. Nat. Neurosci. 6, 989–995 (2003).
Perrett, D. I., Rolls, E. T. & Caan, W. Visual neurones responsive to faces in the monkey temporal cortex. Exp. Brain Res. 47, 329–342 (1982).
Sliwa, J. & Freiwald, W. A. A dedicated network for social interaction processing in the primate brain. Science 356, 745–749 (2017).
Sallet, J. et al. Social network size affects neural circuits in macaques. Science 334, 697–700 (2011).
Baron-Cohen, S., Leslie, A. M. & Frith, U. Does the autistic child have a ‘theory of mind’? Cognition 21, 37–46 (1985).
Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y. & Plumb, I. The ‘Reading the Mind in the Eyes’ Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry Allied Discip. 42, 241–251 (2001).
Constantino, J. N. & Gruber, C. P. Social Responsiveness Scale: Manual (Western Psychological Services, 2005).
Camerer, C. Behavioral Game Theory: Experiments in Strategic Interaction (Russell Sage Foundation, 2003).
Lee, D. & Seo, H. Neural basis of strategic decision making. Trends Neurosci. 39, 40–48 (2016).
Maynard Smith, J. Evolution and the Theory of Games (Cambridge Univ. Press, 1982).
Camerer, C. F. Progress in behavioral game theory. J. Econ. Perspect. 11, 167–188 (1997).
Carlsson, B. & Jönsson, K. I. Differences between the iterated prisoner’s dilemma and the chicken game under noisy conditions. In Proc. 2002 ACM Symposium on Applied Computing—SAC ’02 42–48 (ACM Press, 2002).
Carlsson, B. & Johansson, S. in Agents and Multi-Agent Systems Formalisms, Methodologies, and Applications (eds Wobcke, W. et al) 179–192 (Springer Berlin Heidelberg, 1998).
Crawford, M. P. The Cooperative Solving of Problems by Young Chimpanzees (Johns Hopkins Press, 1938).
Hare, B., Melis, A. P., Woods, V., Hastings, S. & Wrangham, R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623 (2007).
Mendres, K. A. & M De Waal, F. B. Capuchins do cooperate: the advantage of an intuitive task. Anim. Behav. 60, 523–529 (2000).
Drea, C. M. & Carter, A. N. Cooperative problem solving in a social carnivore. Anim. Behav. 78, 967–977 (2009).
Siposova, B., Tomasello, M. & Carpenter, M. Communicative eye contact signals a commitment to cooperate for young children. Cognition 179, 192–201 (2018).
Osborne, M. J. & Rubinstein, A. A Course in Game Theory (MIT Press, 1994).
Samuelson, P. A. Probability, utility, and the independence axiom. Econometrica 20, 670–678 (1952).
Camerer, C. & Ho, T. H. Experience-weighted attraction learning in normal form games. Econometrica 67, 827–874 (1999).
Axelrod, R. & Hamilton, W. D. The evolution of cooperation. Science 211, 1390–1396 (1981).
Nowak, M. & Sigmund, K. A strategy of win–stay, lose–shift that outperforms tit-for-tat in the Prisoner’s Dilemma game. Nature 364, 56–58 (1993).
Sutton, R. S. & Barto, A. G. Reinforcement Learning: An Introduction (MIT Press, 1998).
Chance, M. R. A. Attention structure as the basis of primate rank orders. Man 2, 503–518 (1967).
Shepherd, S. V., Deaner, R. O. & Platt, M. L. Social status gates social attention in monkeys. Curr. Biol. 16, R119–R120 (2006).
Perrett, D. et al. Social signals analyzed at the single cell level: someone is looking at me, something moved! Int. J. Comp. Psychol. 4, 25–55 (1990).
Tsao, D. Y., Freiwald, W. A., Tootell, R. B. H. & Livingstone, M. S. A cortical region consisting entirely of face-selective cells. Science 311, 670–674 (2006).
Hasselmo, M. E., Rolls, E. T. & Baylis, G. C. The role of expression and identity in the face-selective responses of neurons in the temporal visual cortex of the monkey. Behav. Brain Res. 32, 203–218 (1989).
Parkinson, C., Kleinbaum, A. M. & Wheatley, T. Spontaneous neural encoding of social network position. Nat. Hum. Behav. 1, 0072 (2017).
Drea, C. M. & Wallen, K. Low-status monkeys ‘play dumb’ when learning in mixed social groups. Proc. Natl Acad. Sci. USA 96, 12965–12969 (1999).
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001).
Singer, T. et al. Empathic neural responses are modulated by the perceived fairness of others. Nature 439, 466–469 (2006).
Apps, M. A. J. J. & Sallet, J. Social learning in the medial prefrontal cortex. Trends Cogn. Sci. 21, 151–152 (2017).
Mars, R. B., Sallet, J., Neubert, F.-X. & Rushworth, M. F. S. Connectivity profiles reveal the relationship between brain areas for social cognition in human and monkey temporoparietal cortex. Proc. Natl Acad. Sci. USA 110, 10806–10811 (2013).
Platt, M. L., Seyfarth, R. M. & Cheney, D. L. Adaptations for social cognition in the primate brain. Philos. Trans R. Soc. B Biol. Sci. 371, 20150096 (2016).
Cheney, D. L. & Seyfarth, R. M. How Monkeys See the World: Inside the Mind of Another Species (Univ. of Chicago Press, 1990).
Deaner, R. O., Khera, A. V. & Platt, M. L. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr. Biol. 15, 543–548 (2005).
Chang, S. W. C., Winecoff, A. A. & Platt, M. L. Vicarious reinforcement in rhesus macaques (Macaca mulatta). Front. Neurosci. 5, 27 (2011).
Carpenter, B. et al. Stan: a probabilistic programming language. J. Stat. Softw. 76, 1–32 (2017).
Akaike, H. Information theory and an extension of the maximum likelibood principle. In Proc. Second International Symposium on Information Theory (eds Petrov, B. N. & Caski, F. Caski) 267–281 (1973).
Wagenmakers, E.-J. & Farrell, S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 11, 192–196 (2004).
Acknowledgements
We thank the members of Duke University’s Division of Laboratory Animal Resources for their superb animal care. This work was supported by a grant from SFARI (304935, to M.L.P.), and by R01 MH095894, R01 NS088674, R37 MH109728 and R01 MH108627.
Author information
Authors and Affiliations
Contributions
W.S.O. conceived and carried out the experiments and analyzed the data. S.M.-K. designed the strategic learning model. W.S.O. and M.L.P. wrote the manuscript with input from S.M.-K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Ziv Williams and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Monitoring of gaze behavior and trial outcomes.
a, ROIs for M1’s gaze (red) indicated by rectangles drawn around other monkey’s face, other player’s car, and the payout tokens ahead and to the side. All other gaze points directed to the screen are labeled ‘Screen’. b, Proportion of trials resulting in mixed strategy Nash equilibrium when Vstr>Vcoop and pure Nash equilibrium when Vcoop>Vstr. Solid light blue line, high joystick direction signal (90% dot motion coherence) trials; dotted dark blue line, ambiguous joystick direction signal (0% dot motion coherence) trials. Fine lines indicate + - SEM for 4 live monkey pairs (n = 75,630 trials). c, Monkeys look at the most informative stimuli, calibrated by social context. Top panel: Probability M1 looked towards other player’s car (i) or face/face space (computer condition) (ii), aligned to moving dots onset, in live (blue), decoy (green), and computer (black) conditions. Bottom panel: Difference in probability of gaze towards other player’s car (i) or face (ii) on high and ambiguous joystick direction signal (dot motion coherence) trials. All data calculated in 1 ms bins.
Extended Data Fig. 2 Choice outcomes over time and between agency conditions, and the effect of dominance relationships on model fits.
a, Probability of a crash (top panel) or cooperate (lower panel) event over time. Each point represents a bin of 5 trials from all sessions as a function of social context (blue, live; green, decoy; gray, computer. n = 164,259 trials). b, Probability of a crash (top panel) or cooperate (lower panel) event as a function of payout conditions (difference in the number of tokens available straight ahead, Vstr, and cooperate, Vcoop). Red circles/solid lines, high joystick direction signal (90% dot motion coherence) trials; black triangles/dashed lines, ambiguous joystick direction signal (0% dot motion coherence) trials. c, Frequency of deviating as a function of frequency of choosing straight for 4 monkey pairs, segregated by social dominance. For a given pair, number of times they chose deviate (y-axis) or straight (x-axis) are plotted. Symbol fill colors indicate monkey identity; symbol outline colors indicate monkey dyads. Note that mid-ranking monkeys (purple and brown) fall on both sides of the diagonal, suggesting that strategy depends more on relative status than identity. d, Improvements in model fits depend on dominance relationship between monkeys. Models were fit to each monkey’s choices playing specific other monkeys. Y-axis shows increase in AIC/trial when intention was added to model. Within each monkey pair, one was always dominant to the other, and AIC/trials are segregated by relative status. Grey dotted lines connect monkeys playing each other; colors indicate monkey identity.
Extended Data Fig. 3 Location of neural recordings (ACCg & mSTS) and example neurons.
a, Recording locations in ACCg (orange, coronal section) and mSTS (green, coronal and para-sagittal sections). CS, cingulate sulcus; LS, lateral sulcus; A, anterior; P, posterior. A/P distance from inter-aural 0 indicated for coronal sections. b, PSTHs for 2 example ACCg neurons and 2 example mSTS neurons in monkeys playing a live monkey, aligned to moving dots onset (left) and juice delivery (right). Payoff token onset occurs 500 ms before dots onset, cars move 4 s, later and juice delivery occurs 900 ± 100 ms after cars move. Top two rows, example neurons sensitive to payouts (light blue: Vstraight > Vcooperate; dark blue: Vcooperate > Vstraight); bottom two rows, neurons sensitive to joystick direction signal strength (dot motion coherence); dark red, 0% coherence; light red, 90% coherence.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and Supplementary Tables 1–6.
Supplementary Video
Task video showing two players performing the task, with outcomes indicated in the box below and eyetracking of player 1 (yellow dot).
Rights and permissions
About this article
Cite this article
Ong, W.S., Madlon-Kay, S. & Platt, M.L. Neuronal correlates of strategic cooperation in monkeys. Nat Neurosci 24, 116–128 (2021). https://doi.org/10.1038/s41593-020-00746-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-00746-9
This article is cited by
-
The Joint Simon task is not joint for capuchin monkeys
Scientific Reports (2024)
-
Visuo-frontal interactions during social learning in freely moving macaques
Nature (2024)
-
Neural signatures of natural behaviour in socializing macaques
Nature (2024)
-
Neural cognitive signals during spontaneous movements in the macaque
Nature Neuroscience (2023)