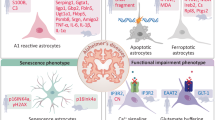
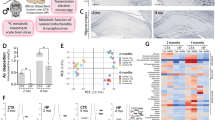
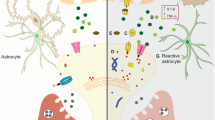
Abstract
Alzheimer’s disease (AD) is characterized by the accumulation of the tau protein in neurons, neurodegeneration and memory loss. However, the role of non-neuronal cells in this chain of events remains unclear. In the present study, we found accumulation of tau in hilar astrocytes of the dentate gyrus of individuals with AD. In mice, the overexpression of 3R tau specifically in hilar astrocytes of the dentate gyrus altered mitochondrial dynamics and function. In turn, these changes led to a reduction of adult neurogenesis, parvalbumin-expressing neurons, inhibitory synapses and hilar gamma oscillations, which were accompanied by impaired spatial memory performances. Together, these results indicate that the loss of tau homeostasis in hilar astrocytes of the dentate gyrus is sufficient to induce AD-like symptoms, through the impairment of the neuronal network. These results are important for our understanding of disease mechanisms and underline the crucial role of astrocytes in hippocampal function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The map sequence for LV construction and microscopy acquisition data have been deposited in Zenodo.org at https://doi.org/10.5281/zenodo.3953694. Source data are provided with this paper.
References
Buee, L. et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 33, 95–130 (2000).
Adams, S. J., de Ture, M. A., McBride, M., Dickson, D. W. & Petrucelli, L. Three repeat isoforms of tau inhibit assembly of four repeat tau filaments. PLoS ONE 5, e10810 (2010).
Panda, D., Samuel, J. C., Massie, M., Feinstein, S. C. & Wilson, L. Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc. Natl Acad. Sci. USA 100, 9548–9553 (2003).
Conrad, C. C. et al. Single-molecule profiling of tau gene expression in Alzheimer’s disease. J. Neurochem. 103, 1228–1236 (2007).
Hamilton, L. K. et al. Aberrant lipid metabolism in the forebrain niche suppresses adult neural stem cell proliferation in an animal model of Alzheimer’s disease. Cell Stem Cell 17, 397–411 (2015).
Santello, M., Toni, N. & Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 22, 154–166 (2019).
Buée-Scherrer, V. et al. AD2, a phosphorylation-dependent monoclonal antibody directed against tau proteins found in Alzheimer’s disease. Mol. Brain. Res. 39, 79–88 (1996).
Kovacs, GaborG. et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 2, 87–102 (2015).
Bereczki, E. et al. Synaptic proteins predict cognitive decline in Alzheimer’s disease and Lewy body dementia. Alzheimers Dement. 12, 1149–1158 (2016).
Savioz, A., Leuba, G. & Vallet, P. G. A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease. Ageing Res. Rev. 18, 86–94 (2015).
Boutajangout, A., Boom, A., Leroy, K. & Brion, J. P. Expression of tau mRNA and soluble tau isoforms in affected and non-affected brain areas in Alzheimer’s disease. FEBS Lett. 576, 183–189 (2004).
Dujardin, S. et al. Neuron-to-neuron wild-type tau protein transfer through a trans-synaptic mechanism: relevance to sporadic tauopathies. Acta Neuropathol. Commun. 2, 14 (2014).
Cheng, Y. & Bai, F. The association of tau with mitochondrial dysfunction in Alzheimer’s disease. Front. Neurosci. 12, 2014–2019 (2018).
Gottlieb, R. A. & Stotland, A. MitoTimer: a novel protein for monitoring mitochondrial turnover in the heart. J. Mol. Med. 93, 271–278 (2015).
Eisner, V., Picard, M. & Hajnóczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 20, 755–765 (2018).
Agarwal, A. et al. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605 (2017).
Nakano, M., Imamura, H., Nagai, T. & Noji, H. Ca2+ regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem. Biol. 6, 709–715 (2011).
Sultan, S. et al. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88, 957–972 (2015).
Crosby, K. C. et al. Nanoscale subsynaptic domains underlie the organization of the inhibitory synapse. Cell Rep. 26, 3284–3297 (2019).
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Pöschel, B., Heinemann, U. & Draguhn, A. High-frequency oscillations in the dentate gyrus of rat hippocampal slices induced by tetanic stimulation. Brain Res. 959, 320–327 (2003).
Towers, S. K. et al. Fast network oscillations in the rat dentate gyrus in vitro. J. Neurophysiol. 87, 1165–1168 (2002).
Espinoza, C., Guzman, S. J., Zhang, X. & Jonas, P. Parvalbumin+ interneurons obey unique connectivity rules and establish a powerful lateral-inhibition microcircuit in dentate gyrus. Nat. Commun. 9, 4605 (2018).
Gillespie, A. K. et al. Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron 90, 740–751 (2016).
Hu, H., Gan, J. & Jonas, P. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263 (2014).
Marissal, T. et al. Restoring wild-type-like CA1 network dynamics and behavior during adulthood in a mouse model of schizophrenia. Nat. Neurosci. 21, 1412–1420 (2018).
LoPresti, P., Szuchet, S., Papasozomenos, S. C., Zinkowski, R. P. & Binder, L. I. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc. Natl Acad. Sci. USA 92, 10369–10373 (1995).
Müller, R., Heinrich, M., Heck, S., Blohm, D. & Richter-Landsberg, C. Expression of microtubule-asssciated proteins MAP2 and tau in cultured rat brain oligodendrocytes. Cell Tissue Res. 288, 239–249 (1997).
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N. & Allen, N. J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285 (2018).
Yamada, K. et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J. Neurosci. 31, 13110–13117 (2011).
Sanders, D. W. et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron https://doi.org/10.1016/j.neuron.2014.04.047 (2014)
Perea, J. R. et al. Extracellular monomeric tau is internalized by astrocytes. Front. Neurosci. 13, 442 (2019).
Dujardin, S. et al. Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS ONE 9, 28–31 (2014).
Goetzl, E. J. et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 30, 3853–3859 (2016).
Wang, Y. & Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016).
Ferrer, I. et al. Aging-related tau astrogliopathy (ARTAG): not only tau phosphorylation in astrocytes. Brain Pathol. 28, 965–985 (2018).
Goode, B. L., Chau, M., Denis, P. E. & Feinstein, S. C. Structural and functional differences between 3-repeat and 4-repeat tau isoforms: implications for normal tau function and the onset of neurodegenerative disease. J. Biol. Chem. 275, 38182–38189 (2000).
Dixit, R., Ross, J. L., Goldman, Y. E. & Holzbaur, E. L. F. Differential regulation of dynein and Kinesin motor proteins by tau. Science 319, 1086–1089 (2010).
Amadoro, G. et al. AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol. Dis. 62, 489–507 (2014).
Yoshiyama, Y., Zhang, B., Bruce, J., Trojanowski, J. Q. & Lee, V. M.-Y. Reduction of detyrosinated microtubules and Golgi fragmentation are linked to tau-induced degeneration in astrocytes. J. Neurosci. 23, 10662–10671 (2003).
van Bergeijk, P., Adrian, M., Hoogenraad, C. C. & Kapitein, L. C. Optogenetic control of organelle transport and positioning. Nature 518, 111–114 (2015).
Forman, M. S. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J. Neurosci. 25, 3539–3550 (2005).
Piacentini, R. et al. Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia 65, 1302–1316 (2017).
Whalley, K. Neurodegenerative disease: spreading the tau. Nat. Rev. Neurosci. 10, 548–548 (2009).
Hainmueller, T. & Bartos, M. Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories. Nat. Rev. Neurosci. 21, 1–16 (2020).
Cope, E. C. & Gould, E. Adult neurogenesis, glia and the extracellular matrix. Cell Stem Cell 24, 690–705 (2019).
Moreno-Jiménez, E. P. et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560 (2019).
Toda, T., Parylak, S. L., Linker, S. B. & Gage, F. H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry https://doi.org/10.1038/s41380-018-0036-2 (2018)
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Bazargani, N. & Attwell, D. Astrocyte calcium signaling: the third wave. Nat. Neurosci. 19, 182–189 (2016).
Braak, H. & Braak, E. Morphology of Alzheimer disease. Fortschr. Med. 108, 624–624 (1990).
Braak, H. & Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278 (1995).
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H. & Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. https://doi.org/10.1007/s00401-006-0127-z (2006).
Flor-García, M. et al. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. 15, 668–693 (2020).
Richetin, K. et al. Amplifying mitochondrial function rescues adult neurogenesis in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 102, 113–124 (2017).
Lee, Y., Messing, A., Su, M. & Brenner, M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56, 481–493 (2008).
De Leeuw, B. et al. Increased glia-specific transgene expression with glial fibrillary acidic protein promoters containing multiple enhancer elements. J. Neurosci. Res. 83, 744–753 (2006).
Merienne, N. et al. Gene transfer engineering for astrocyte-specific silencing in the CNS. Gene Ther. https://doi.org/10.1038/gt.2015.54 (2015)
Colin, A. et al. Engineered lentiviral vector targeting astrocytes in vivo. Glia 57, 667–679 (2009).
Déglon, N. et al. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson’s disease. Hum. Gene Ther. 11, 179–190 (2000).
Sirven, A. et al. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 96, 4103–4110 (2000).
Zufferey, R. et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 72, 9873–9880 (1998).
Lobbestael, E. et al. Immunohistochemical detection of transgene expression in the brain using small epitope tags. BMC Biotechnol. 10, 16 (2010).
Terskikh, A. et al. ‘Fluorescent timer’: protein that changes color with time. Science 290, 1585–1588 (2000).
Hottinger, A. F., Azzouz, M., Déglon, N., Aebischer, P. & Zurn, A. D. Complete and long-term rescue of lesioned adult motoneurons by lentiviral-mediated expression of glial cell line-derived neurotrophic factor in the facial nucleus. J. Neurosci. 20, 5587–5593 (2000).
Kaech, S. & Banker, G. Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415 (2006).
Qi, H. et al. Nuclear magnetic resonance spectroscopy characterization of interaction of tau with DNA and its regulation by phosphorylation. Biochemistry 54, 1525–1533 (2015).
Jicha, G. A., Bowser, R., Kazam, I. G. & Davies, P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 48, 128–132 (1997).
Richetin, K., Petsophonsakul, P., Roybon, L., Guiard, B. P. B. P. & Rampon, C. Differential alteration of hippocampal function and plasticity in females and males of the APPxPS1 mouse model of Alzheimer’s disease. Neurobiol. Aging 57, 220–231 (2017).
Gebara, E. et al. Heterogeneity of radial glia-like cells in the adult hippocampus. Stem Cells 34, 997–1010 (2016).
Stogsdill, J. A. et al. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197 (2017).
Holcomb, L. A. et al. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav. Genet. 29, 177–185 (1999).
Wall, P. & Messier, C. Infralimbic kappa opioid and muscarinic M1 receptor interactions in the concurrent modulation of anxiety and memory. Psychopharmacol. 160, 233–244 (2002).
Ennaceur, A., Neave, N. & Aggleton, J. P. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 113, 509–519 (1997).
Ennaceur, A. & Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 31, 47–59 (1988).
Dodart, J. C., Mathis, C. & Ungerer, A. Scopolamine-induced deficits in a two-trial object recognition task in mice. Neuroreport 8, 1173–1178 (1997).
Richetin, K. et al. Genetic manipulation of adult-born hippocampal neurons rescues memory in a mouse model of Alzheimer’s disease. Brain 138, 440–455 (2015).
Acknowledgements
This study was supported by a Synapsis Foundation fellowship awarded to K.R. and the Lausanne University Hospital (CHUV) and by the Swiss National Science Foundation (31003A_173128 to N.T. and K.R.). L.B., M.C., S.H., R.C. and S.E. were supported by the Programme Investissement d’avenir LabEx (laboratory excellence), DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to ALZheimer’s disease), France Association PSP, the LiCEND (Lille Centre of Excellence in Neurodegenerative Disorders), CNRS, Inserm, Métropole Européenne de Lille, Univ. Lille, FEDER and DN2M. The authors thank the Cellular Imaging Facility of the University of Lausanne for their technical support; F. Magara at the Center for Behavioral Studies of the Lausanne University Hospital, for assistance with the behavioral testing; and H. Imamura of Kyoto University for the MitoGoAteam2 plasmid. We warmly thank C. Rampon and M.C. Miquel at the University of Toulouse and G. Vachey, M. Humbert-Claude, L. Tenenbaum and R. Jenni at the Lausanne University Hospital for their precious help. We also thank S. Sultan, F. Cassé and T. Larrieu for their critical reading of the manuscript and helpful comments.
Author information
Authors and Affiliations
Contributions
K.R. conceived the project and co-supervised the study, acquired and analyzed the data and wrote the manuscript. G.L. collected human samples and performed immunostainings. M.P. and R.P. acquired and analyzed microscopy data. M.M. acquired the data for LV tropism. P.B. designed the calcium imaging experiments. P.S. and K.D. designed and performed the electrophysiology experiments. M.R. cloned the plasmids and produced the LV. C.P., E.P. and R.C. produced the in vitro cultures and immunohistochemistry. S.H., S.B. and M.C. acquired and analyzed data. M.C. and L.B. helped with the research design and critically revised the manuscript. N.T. designed and supervised the study and wrote the manuscript. N.D. designed the LVs and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Differential Aβ accumulation in the hilus of AD patients.
a, Histogram showing the age of patients. b, Histogram showing the post-mortem delay of patients. c, Histogram showing the sex of patients. d, Photomicrographs of the human hippocampus showing the density of Aβ in healthy patient and AD donors. The different areas are indicated as black overlay. e, Histogram showing the density of Aβ in the different hippocampal regions of healthy and AD donors. f, Correlations between Aβ plaques density and Braak stage for patients, for each hippocampal area. g, Table showing the correlation values and P values. Scale bars: 250 µm (d). N = patients/sections per patient; N = 9/4 for Control, N = 6/4 for AD (P-Tau−/Aβ-), N = 6/4 for AD (P-Tau+/Aβ-), N = 9/4 for AD (P-Tau+/Aβ+), (a-c,e-g). One-sided ANOVA with Tukey’s post-hoc test (a-c), Mann-Whitney two-tailed t-test (e) and two-tailed Spearman’s rank non-parametric correlation test (g). Data are presented as the mean ± SEM.
Extended Data Fig. 2 RD3 and RD4 antibodies specificity.
Dot-blot assay to test the specificity of the antibodies raised against 3R tau (RD3, middle panel), 4R tau (RD4, right panel) isoforms of tau or secondary antibody only (left panel). blots is cropped; full gel pictures are shown in supplementary Fig. 2.
Extended Data Fig. 3 Presence of tau isoforms in hilar cells.
a, Confocal micrograph of 3R tau inclusions (red) in a non-astrocytic (s100β-) cell. b, Histogram showing the density of 3R tau inclusions in non-astrocytic cells of CTRL or AD patients. c, Confocal micrograph of 4R tau inclusions (red) in a non-astrocytic cell. d, Histogram showing the density of 4R tau inclusions in non-astrocytic cells. e, Confocal micrographs showing hilar astrocytes (green) that do not contain 3R tau inclusions (red, top panels) or do contain tau 3R inclusions (bottom panels, white arrows). f, Confocal micrographs showing hilar astrocytes (green) that do not contain 4R tau inclusions (red, top panels) or do contain tau 4R inclusions (bottom panels, white arrows). g, Confocal micrographs showing S100β+ astrocytes (green) in the hilus of CTRL and AD donors. h, Histogram showing the density of S100β+ astrocytes in the hilus of CTRL or AD patients. n = patients/sections per patient; N = 9/4 for Control, N = 6/4 for AD (P-Tau−/Aβ-), N = 6/4 for AD (P-Tau+/Aβ-), N = 8/4 for AD (P-Tau+/Aβ+), (b, d, h). One-sided ANOVA with Tukey’s post-hoc test. Data are presented as the mean ± SEM. Scale bars: 10 µm (a, c, e, f) 50 µm (g).
Extended Data Fig. 4 Synaptophysin expression in the hilus of patients.
a, Photomicrographs showing Synaptophysin immunostaining in the hilus of CTRL or AD donors. b, Histogram showing the intensity of Synaptophysin staining in CTRL or AD donors. c, Correlation plot between the intensity of Synaptophysin staining and the number of hilar astrocytes expressing 3R tau in AD patients. d, Correlation plot between the intensity of Synaptophysin staining and the number of hilar astrocytes expressing 4R tau in AD patients. N=patients/sections per patient; N = 9/4 for Control, N = 6/4 for AD (P-Tau−/Aβ-), N = 6/4 for AD (P-Tau+/Aβ-), N = 8/4 for AD (P-Tau+/Aβ+), (b-d). One-sided ANOVA with Tukey’s post-hoc test (b) and two-tailed Spearman’s rank non-parametric correlation test (c, d). Data are presented as the mean ± SEM. Scale bar: 25 µm.
Extended Data Fig. 5 LV-G1-GFP targets a small proportion of RGL stem cells of the dentate gyrus.
a, Confocal micrographs showing an astrocyte (left) and a Radial Glial-Like cell (RGL: right) that expressed GFP, 4 days after intrahippocampal injections of LV-G1-GFP. b, Histogram showing the proportion of infected cells (GFP+) that exhibited the morphology of astrocytes or RGL cells, 4, 14 and 120 days after intrahippocampal injections (dpi) of LV-G1-GFP. c, Confocal micrographs showing a RGL cell expressing GFP and GFAP (red), 4 days after intrahippocampal injections of LV-G1-GFP. Right panels: One channel view of the cell shown on the left panel. d, Histogram showing the proportion of RGL cells expressing GFP, 4, 14 and 120 days after intrahippocampal injections of LV-G1-GFP. N=animals/sections per animal; 4dpi: 6/5, 14dpi:6/5, 120dpi:6/5 (b-d). One-sided ANOVA with Tukey’s post-hoc test (d). Data are presented as the mean ± SEM. Scale bars: 10 µm (a), 50 µm (c).
Extended Data Fig. 6 Triple infection with LV-G1-CFP, LV-G1-1N3R and LV-G1-MitoTimer.
a, Confocal micrographs of the hilus, 120 days after intrahippocampal injections of LV-G1-GFP or LV-G1-1N3R+LV-G1-GFP or LV-G1-1N4R+LV-G1-GFP showing the co-localization of GFP or V5 (green) and tau MC-1 (red). b, Confocal micrograph showing the hilus of the dentate gyrus after infection with the 3 LVs. c, Higher magnification view of the astrocyte highlighted on (b). d, Three channel view of the same cell shown in (b). Scale bars: 10 µm (a), 25 µm (b), 5 µm (c,d).
Extended Data Fig. 7 In vitro targeting of astrocytes and morphological analyses.
a, Confocal micrographs of cultures infected with LV-G1-GFP showing the co-localization of GFP and GFAP (red, left panel), NeuN (red, middle panel) or Iba1 (red, right panel). b, Histogram showing the proportion of infected cells that co-expressed GFP with GFAP, Iba1 or NeuN, c, Confocal micrographs of cultures co-infected with LV-G1-GFP or LV-G1-1N3R+LV-G1-GFP or LV-G1-1N4R+LV-G1-GFP. d, Histogram showing the proportion of cells that were co-infected in the LV-G1-1N3R+LV-G1-GFP or LV-G1-1N4R+LV-G1-GFP conditions. e, Confocal micrographs of astrocytes after infection with LV-G1-GFP or LV-G1-1N3R+LV-G1-GFP or LV-G1-1N4R+LV-G1-GFP. Images are overlaid with a scaffold of the cell’s morphology. f-k, Violin graphs of the astrocytes’ (f) soma area, (g) total territory area, (h) total length of processes, (i) number of branching points, j, number of segments, (k) number of terminal points. N=cultures/cell per culture. (b): LV-G1-CFP: 4/203. (d): LV-G1-1N3R: 4/102 and LV-G1-1N3R: 4/97. (f): LV-G1-CFP: 4/70, LV-G1-1N3R: 4/81 and LV-G1-1N3R: 4/55. (g-k): LV-G1-CFP: 4/12, LV-G1-1N3R: 4/12 and LV-G1-1N3R: 4/12. Data are presented as the mean ± SEM. One-sided ANOVA with Tukey’s post-hoc test (b, f-k) and Mann-Whitney two-tailed t-test (d). Data are presented as the mean ± SEM. Scale bars: 50 µm (c), 20 µm (a, e).
Extended Data Fig. 8 3R tau accumulation in hilar astrocytes does not impact behaviors that are not related to spatial memory.
a, Schematic representation of the object recognition task. b, Histogram of the time spent interacting with the new and old object in animals infected with the LV-G1-GFP (white bars) or LV-G1-1N3R (yellow bars) LV. c, Histogram of the percentage of time spent interacting with the new object. d, Schematic representation of the dark/light box test. e, Histogram showing the time spent in each compartment. f, Schematic representation of the Y-maze. g, Histogram of the spontaneous alterations between each arm. h, Schematic representation of the contextual fear conditioning. (i) Histogram showing the percentage of freezing time before fear conditioning. j, Histogram showing the percentage of freezing time 24H after fear conditioning. LV-G1-CFP, N = 9 mice; LV-G1-1N3R, N = 12 mice. Data are presented as the mean ± SEM. Mann-Whitney two-tailed t-test (b, c, e, g, i, j), Wilcoxon signed-rank test to chance level with ### p < 0.001, ##p < 0.05, #p < 0.01 (c). Data are presented as the mean ± SEM.
Supplementary information
Supplementary Information
Supplementary Table 1 and Supplementary Figs. 1 and 2.
Supplementary Video 1
Example of time-lapse confocal movie showing an astrocyte (left) in a neuron/glial hippocampal co-culture, infected with both LV-G1-CFP (to label the cell; blue) and LV-G1-MitoTimer (to label mitochondria; white). Right: higher magnification movie showing mitochondrial dynamics in different regions of the astrocyte. Scale bar: 10 μm (left); 1 μm (right).
Supplementary Video 2
Example of time-lapse confocal movie showing an astrocyte in a neuron/glial hippocampal co-culture, infected with either LV-G1-CFP and LV-G1-MitoTimer (left) or LV-G1-CFP, LV-G1-1N3R and LV-G1-MitoTimer (right); scale bar: 10 μm.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Fig. 1
Statistical source data.
Source Data Extended Fig. 3
Statistical source data.
Source Data Extended Fig. 4
Statistical source data.
Source Data Extended Fig. 5
Statistical source data.
Source Data Extended Fig. 7
Statistical source data.
Source Data Extended Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Richetin, K., Steullet, P., Pachoud, M. et al. Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat Neurosci 23, 1567–1579 (2020). https://doi.org/10.1038/s41593-020-00728-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-00728-x
This article is cited by
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Astrocytes in the adult dentate gyrus—balance between adult and developmental tasks
Molecular Psychiatry (2024)
-
Targeting vulnerable microcircuits in the ventral hippocampus of male transgenic mice to rescue Alzheimer-like social memory loss
Military Medical Research (2024)
-
Ogt-mediated O-GlcNAcylation inhibits astrocytes activation through modulating NF-κB signaling pathway
Journal of Neuroinflammation (2023)
-
Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation
Journal of Neuroinflammation (2023)