Abstract
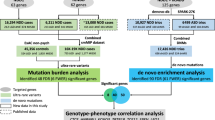
Protein-coding de novo mutations (DNMs) are significant risk factors in many neurodevelopmental disorders, whereas schizophrenia (SCZ) risk associated with DNMs has thus far been shown to be modest. We analyzed DNMs from 1,695 SCZ-affected trios and 1,077 published SCZ-affected trios to better understand the contribution to SCZ risk. Among 2,772 SCZ probands, exome-wide DNM burden remained modest. Gene set analyses revealed that SCZ DNMs were significantly concentrated in genes that were highly expressed in the brain, that were under strong evolutionary constraint and/or overlapped with genes identified in other neurodevelopmental disorders. No single gene surpassed exome-wide significance; however, 16 genes were recurrently hit by protein-truncating DNMs, corresponding to a 3.15-fold higher rate than the mutation model expectation (permuted 95% confidence interval: 1–10 genes; permuted P = 3 × 10−5). Overall, DNMs explain a small fraction of SCZ risk, and larger samples are needed to identify individual risk genes, as coding variation across many genes confers risk for SCZ in the population.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data included in this manuscript have been deposited in the database of Genotypes and Phenotypes (dbGAP) under accession number phs001196.v1.
Data collection and analysis were not performed with blinding to the conditions of the experiments.
Code availability
Code used to identify coding DNMs and assess enrichment is publicly available at https://github.com/howrigan/trio_sequence_analysis.
Change history
24 February 2020
In the version of this article initially published, a string of nonsense characters appears in the second sentence of the section ‘Enrichment in highly brain-expressed and constrained genes’. This has been corrected in the PDF and HTML versions.
References
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Lee, S. H. et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat. Genet. 44, 247–250 (2012).
Power, R. A. et al. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry 70, 22–30 (2013).
Marshall, C. R. et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 49, 27–35 (2017).
Malhotra, D. & Sebat, J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell 148, 1223–1241 (2012).
Rees, E. et al. Analysis of copy number variations at 15 schizophrenia-associated loci. Br. J. Psychiatry 204, 108–114 (2014).
de Ligt, J. et al. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 367, 1921–1929 (2012).
Rauch, A. et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet 380, 1674–1682 (2012).
Lelieveld, S. H. et al. Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat. Neurosci. 19, 1194–1196 (2016).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014).
Iossifov, I. et al. De novo gene disruptions in children on the autistic spectrum. Neuron 74, 285–299 (2012).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014).
Neale, B. M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485, 242–245 (2012).
O’Roak, B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485, 246–250 (2012).
Sanders, S. J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485, 237–241 (2012).
Epi, K. C. et al. De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013).
Epi, K. C. De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am. J. Hum. Genet. 99, 287–298 (2016).
Deciphering Developmental Disorders Study. Large-scale discovery of novel genetic causes of developmental disorders. Nature 519, 223–228 (2015).
Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature 542, 433–438 (2017).
Fromer, M. et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184 (2014).
Genovese, G. et al. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat. Neurosci. 19, 1433–1441 (2016).
Singh, T. et al. The contribution of rare variants to risk of schizophrenia in individuals with and without intellectual disability. Nat. Genet. 49, 1167–1173 (2017).
Singh, T. et al. Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat. Neurosci. 19, 571–577 (2016).
Takata, A. et al. Loss-of-function variants in schizophrenia risk and SETD1A as a candidate susceptibility gene. Neuron 82, 773–780 (2014).
Girard, S. L. et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat. Genet. 43, 860–863 (2011).
Guipponi, M. et al. Exome sequencing in 53 sporadic cases of schizophrenia identifies 18 putative candidate genes. PLoS One 9, e112745 (2014).
Gulsuner, S. et al. Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154, 518–529 (2013).
McCarthy, S. E. et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol. Psychiatry 19, 652–658 (2014).
Xu, B. et al. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat. Genet. 43, 864–868 (2011).
Xu, B. et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat. Genet. 44, 1365–1369 (2012).
Samocha, K. E. et al. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 46, 944–950 (2014).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Karczewski, K. J. et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/531210v3 (2019).
Kosmicki, J. A. et al. Refining the role of de novo protein-truncating variants in neurodevelopmental disorders by using population reference samples. Nat. Genet. 49, 504–510 (2017).
Takata, A. et al. De novo synonymous mutations in regulatory elements contribute to the genetic etiology of autism and schizophrenia. Neuron 89, 940–947 (2016).
Petrovski, S. et al. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 9, e1003709 (2013).
Robinson, E. B. et al. Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc. Natl Acad. Sci. USA 111, 15161–15165 (2014).
Ganna, A. et al. Ultra-rare disruptive and damaging mutations influence educational attainment in the general population. Nat. Neurosci. 19, 1563–1565 (2016).
Purcell, S. M. et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506, 185–190 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012).
Robinson, J. T. et al. Integrative Genomics Viewer. Nat. Biotechnol. 29, 24–26 (2011).
Liu, X., Jian, X. & Boerwinkle, E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum. Mutat. 32, 894–899 (2011).
Liu, X., Jian, X. & Boerwinkle, E. dbNSFPv2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum. Mutat. 34, E2393–E2402 (2013).
Ng, S. B. et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature 461, 272–276 (2009).
Fairbrother, W. G. et al. Predictive identification of exonic splicing enhancers in human genes. Science 297, 1007–1013 (2002).
Ke, S. et al. Quantitative evaluation of all hexamers as exonic splicing elements. Genome Res. 21, 1360–1374 (2011).
Wang, Z. et al. Systematic identification and analysis of exonic splicing silencers. Cell 119, 831–845 (2004).
Acknowledgements
This study was supported by grants from the National Human Genome Research Institute (U54 HG003067 and R01 HG006855), the Stanley Center for Psychiatric Research and the National Institute of Mental Health (R01 MH077139, R01 MH085521 and RC2 MH089905).
Author information
Authors and Affiliations
Contributions
N.L., J.L.M., S.V.F., S.J.G., S.A.M., M.T. and B.M.N. initiated the project. H.-G.H. and W.J.C. led sample recruitment in Taiwan. H.-G.H., W.J.C., S.-H.W., S.V.F., S.J.G., N.L. and M.T. provided the sample and phenotype collection. F.C., K.C., S.D.C., A.D., J.L.M. and C.R. managed the sample collection and processing. D.P.H., K.E.S. and M.F. processed sequence data and generated DNM calls. S.A.R., F.C. and S.A.M. undertook validation of mutations and additional lab work. D.P.H. undertook the main bioinformatics and statistical analyses in close coordination with K.E.S., M.F., G.G., J.A.K., T.S. and B.M.N. The main findings were interpreted by K.E.S., M.F., M.J.D., G.G., J.K., T.S., S.V.F., S.J.G. and B.M.N. D.P.H. drafted the manuscript in close coordination with B.M.N. and C.C., with editing assistance from S.A.R., K.E.S., G.G., J.A.K., S.V.F. and S.J.G.
Corresponding authors
Ethics declarations
Competing interests
B.M.N. is on the Scientific Advisory Board of Deep Genomics and Camp4 Therapeutics Corporation and is on the Biogen Genomics Advisory Panel. M.F. is an employee of Verily Life Sciences.
Additional information
Peer review information Nature Neuroscience thanks Ryan Yuen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–14, Supplementary Tables 1–5 and Supplementary Figs. 1–36.
Supplementary Data 1
Data file descriptions.
Supplementary Data 2
Taiwanese cohort sample list.
Supplementary Data 3
Taiwanese cohort trio list.
Supplementary Data 4
Taiwanese cohort DNM list.
Supplementary Data 5
DNM studies.
Supplementary Data 6
Combined cohorts DNM list.
Supplementary Data 7
SCZ DNM calls.
Supplementary Data 8
Candidate gene sets.
Supplementary Data 9
Candidate gene set results. Summary statistics from candidate gene set enrichment analysis.
Supplementary Data 10
GO + SynaptomeDB enrichment.
Supplementary Data 11
Recurrent PTV genes.
Supplementary Data 12
Recurrent PTV + missense genes.
Supplementary Data 13
Gene recurrence by gene set.
Supplementary Data 14
Predicted SCZ risk genes.
Rights and permissions
About this article
Cite this article
Howrigan, D.P., Rose, S.A., Samocha, K.E. et al. Exome sequencing in schizophrenia-affected parent–offspring trios reveals risk conferred by protein-coding de novo mutations. Nat Neurosci 23, 185–193 (2020). https://doi.org/10.1038/s41593-019-0564-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0564-3
This article is cited by
-
Meta-analysis of 46,000 germline de novo mutations linked to human inherited disease
Human Genomics (2024)
-
An unsupervised deep learning framework for predicting human essential genes from population and functional genomic data
BMC Bioinformatics (2023)
-
Genomic findings in schizophrenia and their implications
Molecular Psychiatry (2023)
-
Clinical characteristics indexing genetic differences in schizophrenia: a systematic review
Molecular Psychiatry (2023)
-
The genetic architecture of schizophrenia: review of large-scale genetic studies
Journal of Human Genetics (2023)