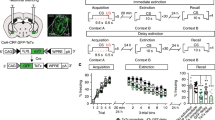
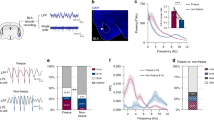
Abstract
Acquisition and extinction of learned fear responses utilize conserved but flexible neural circuits. Here we show that acquisition of conditioned freezing behavior is associated with dynamic remodeling of relative excitatory drive from the basolateral amygdala (BLA) away from corticotropin releasing factor-expressing (CRF+) centrolateral amygdala neurons, and toward non-CRF+ (CRF−) and somatostatin-expressing (SOM+) neurons, while fear extinction training remodels this circuit back toward favoring CRF+ neurons. Importantly, BLA activity is required for this experience-dependent remodeling, while directed inhibition of the BLA–centrolateral amygdala circuit impairs both fear memory acquisition and extinction memory retrieval. Additionally, ectopic excitation of CRF+ neurons impairs fear memory acquisition and facilities extinction, whereas CRF+ neuron inhibition impairs extinction memory retrieval, supporting the notion that CRF+ neurons serve to inhibit learned freezing behavior. These data suggest that afferent-specific dynamic remodeling of relative excitatory drive to functionally distinct subcortical neuronal output populations represents an important mechanism underlying experience-dependent modification of behavioral selection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (2013).
Fadok, J. P. et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).
Douglass, A. M. et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci. 20, 1384–1394 (2017).
Amir, A., Lee, S. C., Headley, D. B., Herzallah, M. M. & Pare, D. Amygdala signaling during foraging in a hazardous environment. J. Neurosci. 35, 12994–13005 (2015).
Han, W. et al. Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 168, 311–324.e18 (2017).
Do Monte, F. H., Quirk, G. J., Li, B. & Penzo, M. A. Retrieving fear memories, as time goes by. Mol. Psychiatry 21, 1027–1036 (2016).
Maren, S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 24, 897–931 (2001).
Duvarci, S. & Pare, D. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980 (2014).
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015).
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010).
Haubensak, W. et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010).
Duvarci, S., Popa, D. & Paré, D. Central amygdala activity during fear conditioning. J. Neurosci. 31, 289–294 (2011).
Sanford, C. A. et al. A central amygdala CRF circuit facilitates learning about weak threats. Neuron 93, 164–178 (2017).
Asok, A. et al. Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol. Psychiatry 23, 914–922 (2018).
McCall, J. G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Kim, J., Zhang, X., Muralidhar, S., LeBlanc, S. A. & Tonegawa, S. Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479.e5 (2017).
Gafford, G. M. & Ressler, K. J. GABA and NMDA receptors in CRF neurons have opposing effects in fear acquisition and anxiety in central amygdala vs. bed nucleus of the stria terminalis. Horm. Behav. 76, 136–142 (2015).
Botta, P. et al. Regulating anxiety with extrasynaptic inhibition. Nat. Neurosci. 18, 1493–1500 (2015).
Yu, K. et al. The central amygdala controls learning in the lateral amygdala. Nat. Neurosci. 20, 1680–1685 (2017).
MacAskill, A. F., Cassel, J. M. & Carter, A. G. Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nat. Neurosci. 17, 1198–1207 (2014).
Nieuwenhuys, R. The insular cortex: a review. Prog. Brain Res. 195, 123–163 (2012).
Berret, E. et al. Insular cortex processes aversive somatosensory information and is crucial for threat learning. Science 364, eaaw0474 (2019).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Cai, H., Haubensak, W., Anthony, T. E. & Anderson, D. J. Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci. 17, 1240–1248 (2014).
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015).
Geddes, S. D. et al. Target-specific modulation of the descending prefrontal cortex inputs to the dorsal raphe nucleus by cannabinoids. Proc. Natl Acad. Sci. USA 113, 5429–5434 (2016).
Biane, J. S., Takashima, Y., Scanziani, M., Conner, J. M. & Tuszynski, M. H. Thalamocortical projections onto behaviorally relevant neurons exhibit plasticity during adult motor learning. Neuron 89, 1173–1179 (2016).
Kaeser, P. S. & Regehr, W. G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 76, 333–363 (2014).
McGarry, L. M. & Carter, A. G. Prefrontal cortex drives distinct projection neurons in the basolateral amygdala. Cell Rep. 21, 1426–1433 (2017).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Anglada-Figueroa, D. & Quirk, G. J. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J. Neurosci. 25, 9680–9685 (2005).
Tipps, M. & Marron Fernandez de Velasco, E. & Schaeffer, A. & Wickman, K. Inhibition of pyramidal neurons in the basal amygdala promotes fear learning. eNeuro 5, ENEURO.0272-18.2018 (2018).
Namburi, P. et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015).
Tervo, D. G. et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92, 372–382 (2016).
Gomez, J. L. et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017).
Gafford, G., Jasnow, A. M. & Ressler, K. J. Grin1 receptor deletion within CRF neurons enhances fear memory. PLoS One 9, e111009 (2014).
Dedic, N. et al. Chronic CRH depletion from GABAergic, long-range projection neurons in the extended amygdala reduces dopamine release and increases anxiety. Nat. Neurosci. 21, 803–807 (2018).
Tovote, P. et al. Midbrain circuits for defensive behaviour. Nature 534, 206–212 (2016).
Evans, D. A. et al. A synaptic threshold mechanism for computing escape decisions. Nature 558, 590–594 (2018).
Pitman, R. K. et al. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 (2012).
Yu, K., Garcia da Silva, P., Albeanu, D. F. & Li, B. Central amygdala somatostatin neurons gate passive and active defensive behaviors. J. Neurosci. 36, 6488–6496 (2016).
van den Pol, A. N. Neuropeptide transmission in brain circuits. Neuron 76, 98–115 (2012).
Yeung, M., Engin, E. & Treit, D. Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology (Berl.) 216, 557–567 (2011).
Yeung, M. & Treit, D. The anxiolytic effects of somatostatin following intra-septal and intra-amygdalar microinfusions are reversed by the selective sst2 antagonist PRL2903. Pharmacol. Biochem. Behav. 101, 88–92 (2012).
Kahl, E. & Fendt, M. Injections of the somatostatin receptor type 2 agonist L-054,264 into the amygdala block expression but not acquisition of conditioned fear in rats. Behav. Brain Res. 265, 49–52 (2014).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008).
McCullough, K. M. et al. Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat. Commun. 7, 13149 (2016).
Madisen, L. et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011).
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Elsevier, 2007).
Hartley, N. D. et al. 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl. Psychiatry 6, e749 (2016).
Acknowledgements
This work was supported by National Institutes of Health grants (nos. MH107435 and AA26186 to S.P., F31 MH111103 to N.D.H. and DA042475 and AA019455 to D.G.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Imaging of ChR2 expression in the CeL and in situ hybridization was performed in part through the use of the Vanderbilt Cell Imaging Shared Resource Center. We thank R. Matthews for training laboratory personnel in the use of confocal microscopes in this study.
Author information
Authors and Affiliations
Contributions
N.D.H. completed all experiments with assistance from A.D.G., R.B., N.D.W., L.E.R.V. and A.J. in the laboratory of S.P. G.J.S. completed the RNA scope studies in the laboratory of D.G.W. N.D.H. and S.P. conceived and designed the studies and wrote the manuscript with input from all coauthors.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests. S.P. has received research contract support from H. Lundbeck A/S unrelated to the current work. S.P is also a scientific consultant for Psy Therapeutics, Sophren Therapeutics and H. Lundbeck A/S, all unrelated to the present work.
Additional information
Peer review information Nature Neuroscience thanks Kai Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of CRF+ neurons in the CeL.
a, Resting membrane potential of neighboring CRF+ and CRF- neurons (n = 17 cells per group, 4 mice; two-tailed unpaired t-test, t(32) = 2.682, P = 0.0115). b, Time constant of neighboring CRF+ and CRF- neurons (n = 17 cells per group; two-tailed unpaired t-test, t(32) = 3.025, P = 0.0049). c, Afterhyperpolarization amplitude of neighboring CRF+ and CRF- neurons (n = 17 cells per group). d, Input resistance of neighboring CRF+ and CRF- neurons (n = 17 cells per group). e, Action potential half-width of neighboring CRF+ and CRF- neurons (n = 17 cells per group; two-tailed unpaired t-test, t(32) = 2.098, P = 0.0439). f, Number of action potentials per current injection of neighboring CRF+ and CRF- neurons (n = 17 cells per group). g, Left: image showing immunohistochemical analysis of PKCδ overlap with CRF+ neurons (scale bar 250 μm). Top-right: close up inlet of image on left. Bottom-right: percentage overlap of CRF+ and PKCδ+ neurons (n = 4 mice). h, Image demonstrating immunohistochemical analysis of PKCδ and CRF+ neurons in the CeA with DAPI stain for quantification of total neurons in CeL. Right: percent of PKCδ+ and CRF+ neurons in the CeL along the rostrocaudal axis (note that PKCδ staining of cell somata was absent in anterior CeL; n = 3 mice; two-way ANOVA, F(1,8) = 80.08, P < 0.0001 for rostrocaudal axis; post-hoc Holm-Sidak’s multiple comparisons, P = 0.0001 for PKCδ and P = 0.0009 for CRF+). i, Images of fluorescent in situ hybridization for CRF, SOM, and PKCδ mRNA in the CeL. Images are pseudocolored for consistency with CRF+ neurons depicted as red throughout figures; in situ hybridization along the rostrocaudal axis was independently repeated in n = 3 mice. j, Percentage overlap of SOM and PKCδ with CRF in the CeL along the rostrocaudal axis (n = 3 mice). k, Injection strategy for targeting fluorophore expression to CRF and SOM neurons in the CeA. l, Representative images of the rostral, middle, and caudal CeA. YFP signal indicates SOM+ neurons, and mCherry signal indicates CRF+ neurons (inlet indicates either the presence or absence of co-localization between YFP and mCherry; scale bars 200 μm); viral expression along the rostrocaudal axis was independently repeated in n = 4 mice. m, Average quantity of CRF+, SOM+, and co-labeled CRF+ /SOM+ neurons in the CeL along the rostrocaudal axis (n = 4 mice). n, Average percentage of co-labeled CRF+ /SOM+ neurons in the CeL along the rostrocaudal axis (n = 4 mice). Action potentials per current injection are presented as mean ± s.e.m., and bar graphs are presented as mean + s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
Extended Data Fig. 2 Fear conditioning and extinction training bidirectionally remodel excitatory input onto SOM+ neurons.
a, Left: image of fluorescent SOM+ neurons in the CeL from a coronal slice used in recordings (pseudocolored in green). Middle: DIC and fluorescent overlay of patch-clamp recording from a SOM+ neuron. Right: recording schematic of SOM+ neuron in the CeL; native fluorescence was verified in n = 3 slices from a single mouse. b, Traces of sEPSC recordings from SOM+ neurons across behavioral conditions (scale bar 100 ms, and 10pA). c, Average sEPSC frequency and amplitude of SOM+ neurons from naïve (basal), fear conditioned, and fear extinction mice (n = 25 basal, 3 mice, n = 27 fear, 4 mice, n = 22 extinction cells, 3 mice; one-way ANOVA, F(2,71) = 0.1380, P = 0.0030; post-hoc Holm-Sidak’s multiple comparisons, basal vs. fear P = 0.0051, fear vs. extinction P = 0.0051). Bar graphs are presented as mean + s.e.m. **P < 0.01.
Extended Data Fig. 3 Conditioning context or CS exposure does not affect BLA-CeL circuit input bias onto CRF+ and CRF- neurons.
a, Traces of maximal oEPSC amplitude from CRF+ (red) and CRF- (black) neuronal pairs across behavioral conditions for stimulation of the BLA-CeL circuit (scale bars 10 ms, 100pA). b, XY graphs depicting skew-plot of maximal oEPSC amplitude from each CRF+ and CRF- neuronal pair for behavioral conditions (n = 15 basal pairs, 4 mice, n = 17 basal context pairs, 4 mice, and n = 15 basal CS+ pairs, 5 mice; extra sum-of-squares F test, F(1,14) = 30.55, P < 0.0001 for basal, F(1,16) = 35.56, P < 0.0001 for basal context, and F(1,14) = 113.2, P < 0.0001 for basal CS+). c, Representation of CRF+ and CRF- maximal oEPSC amplitude ratio (log scale; n = 14 basal pairs, n = 17 basal context, and n = 14 basal CS+ pairs; Kruskal-Wallis test, ns = non-significant, P = 0.5860). XY skew-plots are presented as absolute value. Bar graphs are presented as mean + s.e.m. ****P < 0.0001.
Extended Data Fig. 4 Experience-dependent remodeling of BLA-CeL input bias is associated with postsynaptic alterations but not changes in presynaptic release probability.
a, Traces of AMPAR/NMDAR ratios and PPR from neighboring CRF+ and CRF- neurons following stimulation of the BLA-CeL circuit (scale bars 20 ms, 200pA). b, Representation of CRF+ and CRF- maximal oEPSC amplitude ratio (log scale; n = 20 basal pseudopairs, n = 17 fear pseudopairs, and n = 20 extinction pseudopairs; Kruskal-Wallis test, P < 0.0001; post-hoc Dunn’s multiple comparisons, basal vs. fear P = 0.0025, fear vs. extinction P < 0.0001). c, XY graphs depicting skew-plot of PPR from neighboring CRF+ and CRF- neurons across behavioral conditions (n = 20 basal pseudopairs, 5 mice, n = 17 fear pseudopairs, 5 mice, and n = 20 extinction pseudopairs, 5 mice; extra sum-of-squares F test, F(1,16) = 11.29, P = 0.0040 for fear). d, Representation of the ratio of PPR between neighboring CRF+ and CRF- neurons (log scale; n = 20 basal pairs, n = 17 fear pairs, and n = 20 extinction pairs). e, XY graphs depicting skew-plot of AMPAR/NMDAR from neighboring CRF+ and CRF- neurons across behavioral conditions (n = 20 basal pseudopairs, 5 mice, n = 17 fear pseudopairs, 5 mice, and n = 20 extinction pseudopairs, 5 mice; extra sum-of-squares F test, F(1,19) = 7.374, P = 0.0137 for basal, and F(1,19) = 20.89, P = 0.0002 for extinction). f, Representation of the ratio of AMPAR/NMDAR between neighboring CRF+ and CRF- neurons (log scale; n = 20 basal pseudopairs, n = 17 fear pseudopairs, and n = 20 extinction pseudopairs; Kruskal-Wallis test, P = 0.0013; post-hoc Dunn’s multiple comparisons, fear vs. extinction P = 0.0008, ns = not significant, P = 0.3683). XY skew-plots are presented as absolute value. Bar graphs are presented as mean + s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 5 Fear conditioning and extinction training bidirectionally remodel the BLA-CeL circuit input bias onto CRF+ and SOM+ neurons.
a, Left: optogenetic circuit mapping approach with viral injection. Right: image of YFP and mCherry expression in SOM+ and CRF+ neurons (image is from an animal with no injection of ChR2 expression in the BLA for clarity of fluorophore expression in adjacent CeA; scale bar 175μm); viral expression was independently verified and repeated in n = 4 mice. b, Experimental paradigm for dual patch-clamp recordings from CRF+ and SOM+ neurons in fear conditioned and fear extinguished mice. Bottom-right: DIC and fluorescent overlay image of dual-patch clamp recording from CRF+ and SOM+ pair. c, Traces of maximal oEPSC amplitude from CRF+ (red) and SOM+ (green) neuronal pairs across behavioral conditions for stimulation of the BLA-CeL circuit (scale bars 10 ms, 200pA). d, XY graphs depicting skew-plot of maximal oEPSC amplitude from each CRF+ and SOM+ neuronal pair for behavioral conditions (n = 14 basal pairs, 5 mice, n = 13 fear pairs, 6 mice, and n = 12 extinction pairs, 5 mice; extra sum-of-squares F test, F(1,13) = 47.34, P < 0.0001 for basal, F(1,12) = 5.444, P = 0.0378 for fear, and F(1,11) = 21.16, P = 0.0008 for extinction). e, Representation of CRF+ /CRF- maximal oEPSC amplitude ratio (log scale; n = 14 basal pairs, n = 13 fear pairs, and n = 12 extinction pairs; Kruskal-Wallis test, P = 0.0042; post-hoc Dunn’s multiple comparisons, basal vs. fear P = 0.0091, fear vs. extinction P = 0.0076). XY skew-plots are presented as absolute value. Bar graphs are presented as mean + s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 6 Gαq-coupled-DREADD excites CRF+ neurons but does not mimic the effects of exogenous CRF on glutamatergic transmission.
a, Left: images representing immunohistochemical analysis of immediate early gene c-Fos in CRF-IRES-Cre mice expressing cre-dependent hM3D(Gq)-mCherry in the CeL, followed by systemic administration of VEH or CNO (scale bar 200μm). Right: quantification of c-Fos+ neurons overlapping with hM3D(Gq)-mCherry+ neurons in the CeL (n = 2 mice per group). b, Average time-locked speed trace during early fear memory recall phase for mice injected with VEH or CNO (first 5 CS+ presentations on extinction session 1 day 2 (d2)) from the same mice in experiment presented in Fig. 7f, g (n = 10 mice per group). c, Representative traces of sEPSCs from CeL neurons before (pre) and after (post) VEH or CRF application (scale bars 100 ms, 10pA). d, Effects of VEH or CRF bath application on sEPSC frequency and amplitude over time (gray bar indicates application of CRF or VEH, n = 14 VEH neurons, 5 mice, n = 13 CNO neurons, 5 mice). e, Summary of sEPSC frequency and amplitude after application of VEH or CRF (n = 14 VEH neurons, 5 mice, n = 13 CNO neurons, 5 mice; two-tailed unpaired t-test, t(25) = 3.818, P = 0.0008). f, Representative traces of sEPSCs from CeL neurons before (pre) and after (post) VEH or CNO application in mice expressing hM3D(Gq)-mCherry in CRF+ neurons of the CeL (scale bars 100 ms, 10pA). g, Effects of VEH or CNO bath application on sEPSC frequency and amplitude over time in mice expressing hM3D(Gq)-mCherry in CRF+ neurons of the CeL (gray bar indicates application of CNO or VEH, n = 12 VEH neurons, 5 mice, n = 11 CNO neurons, 5 mice). h, Summary of sEPSC frequency and amplitude after application of VEH or CNO in mice expressing hM3D(Gq)-mCherry in CRF+ neurons of the CeL (n = 12 VEH neurons, 5 mice, n = 11 CNO neurons, 5 mice). Effects of CRF on sEPSC frequency or amplitude over time are presented as mean ± s.e.m., and bar graphs are presented as mean + s.e.m. *P < 0.05, ***P < 0.001.
Extended Data Fig. 7 CRF+ and SOM+ neurons in the CeA signal through long-range and local GABAergic synapses to the PAG and CeL.
a, Recording sites for long-range projections of SOM+ CeA neurons to the PAG. Red dots indicate responsive neurons demonstrating oIPSCs following ChR2 stimulation of axon terminals, and black dots indicate non-responsive neurons. b, Fluorescent images taken from slice recordings demonstrating the presence of ChR2-eYFP terminals in the ventral/ventrolateral PAG and the dorsal/dorsolateral PAG; location of terminals was independently verified and repeated in n = 4 mice. c, Connectivity index indicating percentage of responsive and non-responsive PAG neurons from SOM+ input (n = 56 neurons, 4 mice; RESP = responsive neurons). d, Example trace of time-locked oIPSC recorded from a responsive neuron in the PAG, which was blocked following application of picrotoxin (scale bars 20 ms, 20pA). e, Summary of oIPSCs blocked by picrotoxin in the PAG (n = 8 neurons, 4 mice). f, Recording sites for long-range projections of CRF+ CeA neurons to the PAG. Red dots indicate responsive neurons demonstrating oIPSCs following ChR2 stimulation of axon terminals, and black dots indicate non-responsive neurons. g, Fluorescent images taken from slice recordings demonstrating the presence of ChR2-eYFP terminals in the ventral/ventrolateral PAG and lack of terminals in the dorsal/dorsolateral PAG; location of terminals was independently verified and repeated in n = 4 mice. h, Connectivity index indicating percentage of responsive and non-responsive PAG neurons from CRF+ input (n = 62 neurons, 4 mice; RESP = responsive neurons). i, Example trace of time-locked oIPSC recorded from a responsive neuron in the PAG, which was blocked following application of picrotoxin (scale bars 20 ms, 20pA). j, Summary of oIPSCs blocked by picrotoxin in the PAG (n = 2 neurons, 2 mice). k, Average amplitude of oIPSCs from SOM+ and CRF+ neuronal projections to the PAG (n = 14 neurons, 4 mice for SOM+ input, n = 2 neurons, 4 mice for CRF+ input). l, Example trace of time-locked oIPSC recorded from a SOM- responsive neuron in the CeL, which was blocked following application of picrotoxin (scale bars 20 ms, 50pA). m, Summary of oIPSCs blocked by picrotoxin in the CeL (n = 2 neurons, 2 mice). n, Example trace of time-locked oIPSC recorded from a CRF- responsive neuron in the CeL, which was blocked following application of picrotoxin (scale bars 20 ms, 100pA). o, Summary of oIPSCs blocked by picrotoxin in the CeL (n = 3 neurons, 3 mice). Bar graphs are presented as mean + s.e.m.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11.
Rights and permissions
About this article
Cite this article
Hartley, N.D., Gaulden, A.D., Báldi, R. et al. Dynamic remodeling of a basolateral-to-central amygdala glutamatergic circuit across fear states. Nat Neurosci 22, 2000–2012 (2019). https://doi.org/10.1038/s41593-019-0528-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0528-7
This article is cited by
-
Plastic and stimulus-specific coding of salient events in the central amygdala
Nature (2023)
-
Hippocampus and amygdala fear memory engrams re-emerge after contextual fear relapse
Neuropsychopharmacology (2022)
-
The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum
Nature Neuroscience (2022)
-
The amygdala modulates prepulse inhibition of the auditory startle reflex through excitatory inputs to the caudal pontine reticular nucleus
BMC Biology (2021)
-
Central amygdala micro-circuits mediate fear extinction
Nature Communications (2021)