Abstract
Neuropathic pain can be a debilitating condition with both sensory and affective components, the underlying brain circuitry of which remains poorly understood. In the present study, a basolateral amygdala (BLA)–prefrontal cortex (PFC)–periaqueductal gray (PAG)–spinal cord pathway was identified that is critical for the development of mechanical and thermal hypersensitivity after peripheral nerve injury. It was shown that nerve injury strengthens synaptic input from the BLA onto inhibitory interneurons located in the prelimbic medial PFC, by virtue of reduced endocannabinoid modulation. These augmented synaptic connections mediate a feedforward inhibition of projections from the PFC to the ventrolateral PAG region and its downstream targets. Optogenetic approaches combined with in vivo pharmacology reveal that these BLA–PFC–PAG connections alter pain behaviors by reducing descending noradrenergic and serotoninergic modulation of spinal pain signals. Thus, a long-range brain circuit was identified that is crucial for pain processing and that can potentially be exploited toward targeting neuropathic pain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data for analysis of in vivo calcium measurements will be made available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon request.
Code availability
The code for analysis of in vivo calcium measurements will be made available upon reasonable request.
References
Lorenz, J., Minoshima, S. & Casey, K. L. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126, 1079–1091 (2003).
Apkarian, A. V. et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 24, 10410–10415 (2004).
Devoize, L., Alvarez, P., Monconduit, L. & Dallel, R. Representation of dynamic mechanical allodynia in the ventral medial prefrontal cortex of trigeminal neuropathic rats. Eur. J. Pain. 15, 676–682 (2011).
Seminowicz, D. A. & Moayedi, M. The dorsolateral prefrontal cortex in acute and chronic pain. J. Pain 18, 1027–1035 (2017).
Taylor, J. J., Borckardt, J. J. & George, M. S. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain 153, 1219–1225 (2012).
Seamans, J. K., Lapish, C. C. & Durstewitz, D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox. Res. 14, 249–262 (2008).
Zhang, Z. et al. Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic. Pain Cell Rep. 12, 752–759 (2015).
Lee, M. et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci. 35, 5247–5259 (2015).
Little, J. P. & Carter, A. G. Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J. Neurosci. 33, 15333–15342 (2013).
McGarry, L. M. & Carter, A. G. Prefrontal cortex drives distinct projection neurons in the basolateral amygdala. Cell Rep. 21, 1426–1433 (2017).
Cheriyan, J., Kaushik, M. K., Ferreira, A. N. & Sheets, P. L. Specific targeting of the basolateral amygdala to projectionally defined pyramidal neurons in prelimbic and infralimbic cortex. eNeuro 3, https://doi.org/10.1523/ENEURO.0002-16.2016 (2016).
Collins, D. P., Anastasiades, P. G., Marlin, J. J. & Carter, A. G. Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron 98, 366–379 (2018).
Cheriyan, J. & Sheets, P. L. Altered excitability and local connectivity of mPFC-PAG neurons in a mouse model of neuropathic pain. J. Neurosci. 38, 4829–4839 (2018).
Woodhams, S. G., Chapman, V., Finn, D. P., Hohmann, A. G. & Neugebauer, V. The cannabinoid system and pain. Neuropharmacology 124, 105–120 (2017).
Fortin, D. A. & Levine, E. S. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb. Cortex 17, 163–174 (2007).
Caballero, A. & Tseng, K. Y. Association of cannabis use during adolescence, prefrontal CB1 receptor signaling, and schizophrenia. Front. Pharmacol. 3, 101 (2012).
Song, J. et al. Crucial role of feedback signals from prelimbic cortex to basolateral amygdala. Sci. Adv. 5, eaat3210 (2019).
Reynolds, D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164, 444–445 (1969).
Basbaum, A. I. & Fields, H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 7, 309–338 (1984).
Samineni, V. K. et al. Divergent modulation of nociception by glutamatergic and GABAergic neuronal subpopulations in the periaqueductal gray. eNeuro 4, https://doi.org/10.1523/ENEURO.0129-16.2017 (2017).
Hardy, S. G. & Leichnetz, G. R. Frontal cortical projections to the periaqueductal gray in the rat a retrograde and orthograde. Neurosci. Lett. 23, 13–17 (1981).
An, X., Bandler, R., Ongür, D. & Price, J. L. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J. Comp. Neurol. 401, 455–479 (1998).
Ferreira, A. N., Yousuf, H., Dalton, S. & Sheets, P. L. Highly differentiated cellular and circuit properties of infralimbic pyramidal neurons projecting to the periaqueductal gray and amygdala. Front. Cell Neurosci. 9, 161 (2015).
Franklin, T. B. et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat. Neurosci. 20, 260–270 (2017).
Rozeske, R. R. et al. Prefrontal-periaqueductal gray-projecting neurons mediate context fear discrimination. Neuron 97, 898–910 (2018). e896.
Kim, J. H. et al. Yin-and-yang bifurcation of opioidergic circuits for descending analgesia at the midbrain of the mouse. Proc. Natl Acad. Sci. USA 115, 11078–11083 (2018).
Ossipov, M. H., Dussor, G. O. & Porreca, F. Central modulation of pain. J. Clin. Invest. 120, 3779–3787 (2010).
Nam, H. & Kerman, I. A. Distribution of catecholaminergic presympathetic-premotor neurons in the rat lower brainstem. Neuroscience 324, 430–445 (2016).
Craig, A. D., Bushnell, M. C., Zhang, E. T. & Blomqvist, A. A thalamic nucleus specific for pain and temperature sensation.pdf. Nature 372, 770–773 (1994).
Johansen, J. P., Fields, H. L. & Manning, B. H. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl Acad. Sci. USA 98, 8077–8082 (2001).
Bliss, T. V., Collingridge, G. L., Kaang, B. K. & Zhuo, M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 17, 485–496 (2016).
Ohara, P. T., Vit, J. P. & Jasmin, L. Cortical modulation of pain. Cell. Mol. Life Sci. 62, 44–52 (2005).
Barthas, F. et al. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol. Psychiatry 77, 236–245 (2015).
Jahn, A., Nee, D. E., Alexander, W. H. & Brown, J. W. Distinct regions within medial prefrontal cortex process pain and cognition. J. Neurosci. 36, 12385–12392 (2016).
Weizman, L. et al. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology 91, 1285–1294 (2018).
Chen, T. et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun. 9, 1886 (2018).
Liu, Y. et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 561, 547–550 (2018).
Ji, G. et al. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 30, 5451–5464 (2010).
Roy, M. et al. Representation of aversive prediction errors in the human periaqueductal gray. Nat. Neurosci. 17, 1607–1612 (2014).
Tinnermann, A., Geuter, S., Sprenger, C., Finsterbusch, J. & Büchel, C. Interactions between PFC-PAG-spinal cord mediate value effects in nocebo hyperalgesia. Science 358, 105–108 (2017).
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015).
Allen, W. E. et al. Thirst-associated preoptic neurons encode an aversive motivational drive. Science 357, 1149–1155 (2017).
Beyeler, A. et al. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron 90, 348–361 (2016).
Zhang, F. X., Gadotti, V. M., Souza, I. A., Chen, L. & Zamponi, G. W. BK potassium channels suppress Cavalpha2delta subunit function to reduce inflammatory and neuropathic pain. Cell Rep. 22, 1956–1964 (2018).
Brenner, D. S., Golden, J. P. & Gereau, R. W. A novel behavioral assay for measuring cold sensation in mice. PLoS One 7, https://doi.org/10.1371/journal.pone.0039765.g001 (2012).
Sestakova, N., Puzserova, A., Kluknavsky, M. & Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 6, 126–135 (2013).
Simone, K., Fuzesi, T., Rosenegger, D., Bains, J. & Murari, K. Open-source, cost-effective system for low-light in vivo fiber photometry. Neurophotonics 5, 025006 (2018).
Huang, J. et al. Targeting the D series resolvin receptor system for the treatment of osteoarthritis pain. Arthr. Rheumatol. 69, 996–1108 (2017).
Acknowledgements
This work was supported by a Foundation Grant to G.W.Z. from the Canadian Institutes of Health Research, and by the Canada–Israel Health Research Initiative, jointly funded by the Canadian Institutes of Health Research, the Israel Science Foundation, the International Development Research Centre and the Azrieli Foundation. G.W.Z. is a Canada Research Chair in Molecular Neuroscience. V.M.G. is supported through the Vi Riddell Program in Pediatric Pain of the Alberta Children’s Hospital Research Institute. S.H. is supported by a studentship from Alberta Innovates and a University of Calgary Eyes-High studentship. We thank T. Fuzesi and the Cumming School of Medicine Optogenetics facility for technical assistance with fiber photometry.
Author information
Authors and Affiliations
Contributions
Z.Z., J.H. and G.W.Z. conceived the project. J.H., Z.Z. and V.M.G. designed and performed the experiments, analyzed the data, prepared the figures and contributed to the writing. L.C. performed the tissue extractions and immunostaining experiments. I.A.S. performed the western blotting. Z.Z., D.W. and S.H. performed the electrophysiology experiments. G.W.Z. directed and supervised the study and co-wrote the manuscript. C.R. and K.D. provided the reagents and discussion, and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nature Neuroscience thanks C. Woolf and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Analysis of nerve injury-induced changes within the endocannabinoid system in the prelimbic area of the mPFC.
a-d, Quantitative PCR analysis of the expression of enzymes involved in endocannabinoid metabolism in the prelimbic area of the mPFC. mRNA levels of NAPE-PLD (a), FAAH (b), MAGL (c) and DAGL (d) in the prelimbic contralateral mPFC at 2 weeks and 4 weeks after Sham and SNI surgery (p = 0.0235, t = 2.489 for 4 weeks in a and all other comparisons are p > 0.1). Data were normalized to β-actin mRNA levels and are presented as mean ± SEM. Two-tailed unpaired t-tests were used for (a-d). Numbers in parentheses reflect numbers of mice for all panels. Asterisks denote significant differences. e, Uncropped Western blot corresponding to the data in main Fig. 1k.
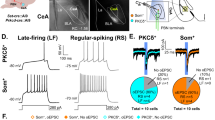
Supplementary Figure 2 Expression and functional effects of Arch3.0 in the BLA.
a, b, Sample images showing eYFP expression in the BLA (a) and the prelimbic mPFC (b) after mice were injected with AAV-CaMKIIα-Arch3.0-eYFP in the BLA (n = 2 mice). The scale bars in (a) and (b) are 200 μm and 50 μm, respectively. c, Sample current-clamp trace showing that yellow light illumination inhibits electrical-evoked action potential firing in an Arch3.0-eYFP expressing BLA neuron (n = 4 cells). d, Sample voltage-clamp traces showing yellow light inhibition of electrical-evoked EPSCs in pyramidal cells of the prelimbic mPFC in mice that are injected with AAV-CaMKIIα-Arch3.0-eYFP or AAV-CaMKIIα-NpHR3.0-eYFP in the BLA. e, Mean eEPSC amplitudes for the experiments in (d) (p = 0.0187, t = 3.429 and p = 0.0004, t = 8.464 for Arch and NpHR, respectively). Note that 2-3 times the threshold stimulus intensity was used to induce eEPSCs and bicuculline (10 μM) was added to the bath solution to isolate direct monosynaptic glutamatergic inputs from the BLA to mPFC pyramidal neurons without incorporating effects of yellow light on GABAergic feedforward inhibition of pyramidal cell activity. Data are presented as mean ± SEM. A two-tailed paired t-test was used for e, where numbers in parentheses denote numbers of cells.
Supplementary Figure 3 Optogenetic manipulation of BLA inputs into the prelimbic mPFC in sham and SNI mice.
a, b, Mechanical paw withdrawal threshold (a) and thermal paw withdrawal latency (b) are not altered by blue light stimulation of BLA inputs into the prelimbic mPFC in Sham mice that were injected with AAV-CaMKIIα-ChR2-eYFP in the BLA (p = 0.8133, F = 0.3165 for a and p = 0.7105, F= 0.4654 for b). c, d, Mechanical paw withdrawal threshold (c) and thermal paw withdrawal latency (d) are unaltered by yellow light stimulation in Sham mice that were injected with AAV5-CaMKIIα-Arch3.0-eYFP in the BLA (p = 0.7606, F= 0.3925 for b and p = 0.5236, F = 0.7876, for d). e, f, g and h, Mechanical paw withdrawal threshold (e) and thermal paw withdrawal latency (f) of the ipsilateral hindpaw are increased following yellow light illumination of the BLA inputs into the mPFC in SNI mice (e, f) injected with AAV-CaMKIIα-NpHR3.0-eYFP in the BLA (p < 0.0001, F = 70.07 for e, p = 0.0045, t = 3.306 for Ipsi vs Contra groups; p = 0.0231, t = 2.804 for Light off vs Light on for Ipsi groups in f) but not in Sham operated animals (g, h) (p = 0.4715, F = 0.8731 for g and p = 0.5509, F = 0.7214 for h). Data are presented as mean ± SEM. We used One-way analysis of variance (ANOVA) with Newman-Keuls comparisons test for (a-e and g-h), a two-tailed unpaired t-test (Contra vs Ipsi) and a paired t-test (Light off vs Light on for Ipsi groups) for f. Numbers in parentheses reflect numbers of mice for all panels. Contra: Contralateral. Ipsi: Ipsilateral. ns: not significant.
Supplementary Figure 4 Motor activity in sham and SNI mice with yellow light stimulation of BLA inputs into the mPFC.
Number of midline crossings in PEA experiments in sham and SNI mice, showing that yellow light illumination of the BLA input into the mPFC does not cause any change in locomotor activity (p = 0.9185, F = 0.1642). Data are presented as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) with Newman-Keuls comparisons test. Numbers in parentheses reflect numbers of mice. ns: not significant.
Supplementary Figure 5 Optogenetic modulation of BLA inputs alters c-fos expression in the prelimbic mPFC.
a, C-fos expression and colocalization (arrows) with parvalbumin (PV) in layer 5 of the prelimbic mPFC of SNI mice without (upper panels) and with (lower panels) blue light activation of BLA-originating ChR2-eYFP expressing terminals in the mPFC. b, Quantification of activated PVINs (colocalization with c-fos) from SNI (upper) and Sham mice (lower) with blue light stimulation (p = 0.0006, F = 14.10 for SNI; p = 0.7372, F = 0.43 for sham). c, C-fos expression and colocalization (arrows) with CaMKIIα in layer 5 of the prelimbic mPFC of SNI mice without (upper panels) and with (lower panels) yellow light inhibition of the BLA-originating Arch3.0-eYFP expressing terminals in the mPFC. d, Quantification of activated PNs (colocalization with c-fos) in the mPFC with yellow light inhibition of BLA inputs (p = 0.0017, F = 15.7). Data are presented as mean ± SEM. One-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons test was used for both (b) and (d). Numbers in parentheses indicate numbers of mice in b and d. Scale bars, 20 μm. PrL: prelimbic, IL: infralimbic.
Supplementary Figure 6 Optogenetic manipulation of BLA-PFC inputs does not affect anxiety behaviors in SNI mice.
a, Number of crossings in sham and SNI mice after ChR2 mediated (blue light) activation of BLA inputs into the mPFC (p = 0.1048, F = 2.82). b, Ratio of time spent in the periphery and center of the open field after blue light stimulation (p = 0.0134, F = 0.014). c, Number of crossings in sham and SNI mice after Arch3.0 mediated (yellow light) inhibition of BLA inputs into the mPFC (p = 0.6988, F = 0.4825). d, Ratio of time spent in the periphery and the center of the open field after yellow light stimulation (p = 0.0046, F = 6.278). Nerve injury reduced the time spent in the center, which is reflective on increased anxiety of SNI mice, but this effect was not altered by optogenetic manipulation (b and d). Data are presented as mean ± SEM. *P < 0.05. One-way analysis of variance (ANOVA) with Newman-Keuls comparisons test for (a-d). Numbers in parentheses indicate numbers of mice for all panels.
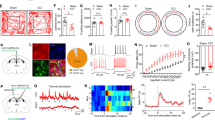
Supplementary Figure 7 Circuit projection from the prelimbic mPFC to PAG subregions.
a, AAV-CaMKIIα-ChR2 (H134R)-eYFP virus was injected in the prelimbic mPFC and YFP fluorescence was examined in various PAG subregions. The mPFC projects mainly to the vlPAG (see main text Fig. 3a) with few projections to the dmPAG, dlPAG and lPAG (n = 4 mice). Scale bar: 50 μm. dm: dorsomedial, dl: dorsolateral, l: lateral. b, Green retrobeads were injected into the vlPAG and were traced back to layer 5 of the prelimbic mPFC (see arrows, n = 3 mice). Scale bars: 200 μm (Left) and 20 μm (Right). c, AAV9-hEF1α-DIO-synaptophysin-mCherry virus was injected in the prelimbic area of mPFC and Cav2 virus was injected in the vlPAG (left). Synaptophysin terminals from mPFC innervate both glutamatergic and GABAergic neurons in the vlPAG as seen from overlap with CamKIIα and GAD67 staining (see arrows, n = 3 mice) (right). Scale bar: 20 μm.
Supplementary Figure 8 Blue light activation of the infralimbic mPFC does not alter nociceptive responses.
a, b, Mechanical paw withdrawal threshold is significantly decreased in the ipsilateral paw compared to the contralateral side in SNI mice (a, ****p < 0.0001, F = 54.03) but not sham mice (b, p = 0.5585, F = 0.721). Furthermore, blue light stimulation of the infralimbic mPFC does not alter nociceptive responses in either SNI (a) or Sham mice (b). Data are presented as mean ± SEM. One-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons test for (a) and (b). Numbers in parentheses denote numbers of mice for all panels. ns: not significant.
Supplementary Figure 9 Effects of optogenetic manipulation of the mPFC–vlPAG circuit on c-fos expression in the vlPAG of SNI mice.
a, b, Colocalization (see arrows) of c-fos with CaMKIIα (a, n = 4 mice) and GAD67 (b, n = 3 mice for no light and n = 4 mice for blue light) in the vlPAG without (upper panel) and with (lower panel) blue light stimulation of mPFC inputs. Scale bar: 20 μm. c, d, Quantification for the percentage of activated glutamatergic (c, p = 0.0106, t = 3.654) and GABAergic (d, p = 0.0205, t = 3.334) neurons in the vlPAG following ChR2 activation. Data are presented as mean ± SEM. A two-tailed unpaired t-test was used for (c) and (d). Numbers in parentheses denote numbers of mice for c and d.
Supplementary Figure 10 Effect of optogenetic manipulation of the BLA–mPFC circuit on c-fos expression in the vlPAG of SNI mice.
a, b, C-fos expression and colocalization (arrows) with CaMKIIα in the vlPAG without (a, n = 3 mice) and with (b, n = 4 mice) yellow light manipulation of the BLA-mPFC circuitry. Scale bar, 20 μm. c, Quantification of the percentage of activated glutamatergic neurons (p = 0.0235, t = 3.220). Data are presented as means ± SEM. Two-tailed unpaired t-test was used for (c). Numbers in parentheses denote numbers of mice for c.
Supplementary Figure 11 Proposed mechanism for signal processing in the vlPAG.
a, b, Bath application of the GABAB receptor agonist baclofen (20-30 μM) consistently induced an outward current in voltage clamp recordings of GABA neurons in the vlPAG (a, p = 0.0010, t = 5.441), and a decrease in membrane input resistance (b, p = 0.0157, t = 3.335). c, Representative trace of a current-clamp recording of GABA neurons in the vlPAG showing that baclofen application induced membrane hyperpolarization. d, A proposed model of possible signal processing in the vlPAG. Blue light stimulation of ChR2 expressing mPFC-vlPAG glutamatergic projections leads to increased activity of glutamatergic projection neurons in the vlPAG, thus boosting glutamatergic outputs. The presumed first order GABA neurons also receive direct light-evoked glutamatergic synaptic input. The first order GABA neurons may inhibit second order GABA neurons via GABAB receptor-mediated volume transmission, thus leading to an overall decrease in c-fos expression in GABA neurons of the vlPAG and reduced GABAergic output to the LC and RVM, therefore, causing analgesia. Data are presented as means ± SEM. A two-tailed paired t-test was used for a and b, with numbers in parentheses reflecting numbers of cells. GABAN: GABA neurons. GABA-b R: GABAB receptor.
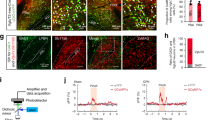
Supplementary Figure 12 GCaMP6f-tdTomato-labeled neurons in the vlPAG, and mapping of vlPAG–LC and vlPAG–RVM projections.
a, Schematic diagram showing the configuration for in vivo fiber photometry to record calcium signals in the vlPAG with yellow light inhibition of the BLA-mPFC circuit. b, Cre-dependent labeling of GABA neurons in the vlPAG expressing a bicystronic AAV-GCaMP6f-tdTomato construct (n = 4 mice), scale bar: 50μm. c, Retrograde tracing of vlPAG-LC projections. CTB488 was injected into the LC and colocalization (arrows) of CTB488 (green) with tdTomato-labeled (red) GABAergic neurons in the vlPAG from GAD2-cre::Ai9 (tdTomato) mice was analyzed (top row) (n = 3 mice). Similarly colocalization of CTB488 with tdTomato-labeled glutamatergic neurons in the vlPAG from Vglut2 cre::Ai9 (tdTomato) mice was tested (bottom row) (n = 6 mice).The scale bar for the LC image is 500 μm and the others are 50 μm. d, Retrograde tracing of vlPAG-RVM projections. CTB488 was injected into the RVM and colocalization (arrows) of CTB488 with tdTomato-labeled GABAergic neurons in the vlPAG from GAD2-cre::Ai9 (tdTomato) mice was examined (top row) (n = 4 mice), as was colocalization of CTB488 with tdTomato-labeled glutamatergic neurons in the vlPAG from Vglut2 cre::Ai9 (tdTomato) mice (bottom row) (n = 3 mice). The scale bar for the LC and RVM image is 500 μm and the others are 50 μm.
Supplementary Figure 13 Lack of effect of intrathecal delivery of noradrenergic and serotoninergic receptor antagonists on mechanical paw withdrawal threshold in SNI mice.
Paw withdrawal thresholds in SNI mice following intrathecal delivery of the 5-HT3 antagonist Ondansetron (p = 0.3419, t = 1.019), the 5HT1/2 antagonist Metergoline (p = 0.5851, t = 0.5721) or the noradrenergic α2A/B antagonist BRL-44408 (p = 0.3775, t = 0.9420). Note that these antagonists do not further exacerbate mechanical hypersensitivity, presumably since descending noradrenergic and serotonergic modulation is already weakened in SNI mice. In contrast, metergoline and BRL-44408 prevent optogenetically induced analgesia (see Fig. 5f). Data are presented as mean ± SEM. A two-tailed unpaired t-test was used for all comparisons between vehicle and drug. Numbers in parentheses reflect numbers of mice.
Supplementary Figure 14 Quantification of neuronal loss in the LC after intrathecal injection of DSP4.
a, b, Immunostaining for TH-positive neurons in the left and right LC after intrathecal (L5) delivery of either PBS or DSP4 (n = 4 mice for both groups), scale bar: 50 μm. c, Quantification of the average numbers of TH-positive neurons in the LC (p = 0.0001, F = 17). The incomplete neuronal loss is expected since the LC does not only project to the lumbar region. Data are presented as means ± SEM. One-way analysis of variance (ANOVA) with Bonferroni’s correction for multiple comparisons was used for (c). Numbers in parentheses reflect numbers of mice in c. L: left, R: right, LC: locus coeruleus.
Supplementary Figure 15 Model summarizing key experimental findings.
Top: Cartoon depicting putative ascending and descending pathways linking to the BLA-mPFC-PAG circuit described in this manuscript. Dashed lines indicate projections that were not explicitly examined in this study. We hypothesize that after peripheral nerve injury, ascending projections from the spinal cord via the parabrachial nucleus (PBN) send pain signals to the BLA. Increased inputs of amygdala projection neurons into PVINs of the mPFC lead to enhanced feedforward inhibition of mPFC Layer 5 pyramidal neurons, leading to hypoactivity and thus reduced inputs into the vlPAG. This in turn leads to reduced noradrenergic or serotonergic descending modulation of spinal pain circuits, possibly via the LC or RVM. Optogenetic inhibition of BLA-mPFC projections reverses this process, leading to pain relief. Bottom: Alternative representation of the scheme depicted in the top panel.
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, J., Gadotti, V.M., Chen, L. et al. A neuronal circuit for activating descending modulation of neuropathic pain. Nat Neurosci 22, 1659–1668 (2019). https://doi.org/10.1038/s41593-019-0481-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0481-5
This article is cited by
-
Direct paraventricular thalamus-basolateral amygdala circuit modulates neuropathic pain and emotional anxiety
Neuropsychopharmacology (2024)
-
A distinct cortical code for socially learned threat
Nature (2024)
-
A mesocortical glutamatergic pathway modulates neuropathic pain independent of dopamine co-release
Nature Communications (2024)
-
Neural circuits regulating visceral pain
Communications Biology (2024)
-
Amygdala-Targeted Relief of Neuropathic Pain: Efficacy of Repetitive Transcranial Magnetic Stimulation in NLRP3 Pathway Suppression
Molecular Neurobiology (2024)