Abstract
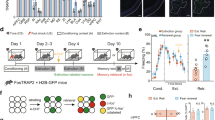
Learned fear often relapses after extinction, suggesting that extinction training generates a new memory that coexists with the original fear memory; however, the mechanisms governing the expression of competing fear and extinction memories remain unclear. We used activity-dependent neural tagging to investigate representations of fear and extinction memories in the dentate gyrus. We demonstrate that extinction training suppresses reactivation of contextual fear engram cells while activating a second ensemble, a putative extinction engram. Optogenetic inhibition of neurons that were active during extinction training increased fear after extinction training, whereas silencing neurons that were active during fear training reduced spontaneous recovery of fear. Optogenetic stimulation of fear acquisition neurons increased fear, while stimulation of extinction neurons suppressed fear and prevented spontaneous recovery. Our results indicate that the hippocampus generates a fear extinction representation and that interactions between hippocampal fear and extinction representations govern the suppression and relapse of fear after extinction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Powers, M. B., Halpern, J. M., Ferenschak, M. P., Gillihan, S. J. & Foa, E. B. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin. Psychol. Rev. 30, 635–641 (2010).
Milad, M. R. & Quirk, G. J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151 (2012).
Rescorla, R. A. Spontaneous recovery. Learn. Mem. 11, 501–509 (2004).
Pavlov, I. P. Conditioned Reflexes (Oxford Univ. Press, 1927).
Bouton, M. E., Westbrook, R. F., Corcoran, K. A. & Maren, S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol. Psychiatry 60, 352–360 (2006).
Myers, K. M. & Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 12, 120–150 (2007).
Tovote, P., Fadok, J. P. & Lüthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015).
Trouche, S., Sasaki, J. M., Tu, T. & Reijmers, L. G. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 80, 1054–1065 (2013).
Bernier, B. E. et al. Dentate gyrus contributes to retrieval as well as encoding: evidence from context fear conditioning, recall, and extinction. J. Neurosci. 37, 6359–6371 (2017).
Corcoran, K. A. & Maren, S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 21, 1720–1726 (2001).
Corcoran, K. A. & Maren, S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn. Mem. 11, 598–603 (2004).
Hobin, J. A., Ji, J. & Maren, S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus 16, 174–182 (2006).
Denny, C. A. et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014).
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012).
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013).
Ryan, T. J., Roy, D. S., Pignatelli, M., Arons, A. & Tonegawa, S. Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013 (2015).
Redondo, R. L. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014).
Kitamura, T. et al. Engrams and circuits crucial for systems consolidation of a memory. Science 356, 73–78 (2017).
Roy, D. S. et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531, 508–512 (2016).
Cazzulino, A. S., Martinez, R., Tomm, N. K. & Denny, C. A. Improved specificity of hippocampal memory trace labeling. Hippocampus 26, 752–762 (2016).
Sun, Q. et al. Proximodistal heterogeneity of hippocampal CA3 pyramidal neuron intrinsic properties, connectivity, and reactivation during memory recall. Neuron 95, 656–672.e3 (2017).
Tayler, K. K., Tanaka, K. Z., Reijmers, L. G. & Wiltgen, B. J. Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol. 23, 99–106 (2013).
Cai, D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016).
Reijmers, L. G., Perkins, B. L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007).
Tanaka, K. Z. et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84, 347–354 (2014).
Fanselow, M. S. Contextual fear, gestalt memories, and the hippocampus. Behav. Brain Res. 110, 73–81 (2000).
Rudy, J. W. & O’Reilly, R. C. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn. Affect. Behav. Neurosci. 1, 66–82 (2001).
O’Reilly, R. C. & Rudy, J. W. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol. Rev. 108, 311–345 (2001).
Rudy, J. W., Huff, N. C. & Matus-Amat, P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci. Biobehav. Rev. 28, 675–685 (2004).
Rudy, J. W., Barrientos, R. M. & O’Reilly, R. C. Hippocampal formation supports conditioning to memory of a context. Behav. Neurosci. 116, 530–538 (2002).
Wang, M. E., Yuan, R. K., Keinath, A. T., Ramos Álvarez, M. M. & Muzzio, I. A. Extinction of learned fear induces hippocampal place cell remapping. J. Neurosci. 35, 9122–9136 (2015).
Tronson, N. C. et al. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J. Neurosci. 29, 3387–3394 (2009).
Kheirbek, M. A. et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968 (2013).
Lassalle, J.-M., Bataille, T. & Halley, H. Reversible inactivation of the hippocampal mossy fiber synapses in mice impairs spatial learning, but neither consolidation nor memory retrieval, in the Morris navigation task. Neurobiol. Learn. Mem. 73, 243–257 (2000).
Madroñal, N. et al. Rapid erasure of hippocampal memory following inhibition of dentate gyrus granule cells. Nat. Commun. 7, 10923 (2016).
Khalaf, O. et al. Reactivation of recall-induced neurons contributes to remote fear memory attenuation. Science 360, 1239–1242 (2018).
Corcoran, K. A., Leaderbrand, K. & Radulovic, J. Extinction of remotely acquired fear depends on an inhibitory NR2B/PKA pathway in the retrosplenial cortex. J. Neurosci. 33, 19492–19498 (2013).
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008).
Grewe, B. F. et al. Neural ensemble dynamics underlying a long-term associative memory. Nature 543, 670–675 (2017).
Milad, M. R. & Quirk, G. J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74 (2002).
Sotres-Bayon, F., Sierra-Mercado, D., Pardilla-Delgado, E. & Quirk, G. J. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76, 804–812 (2012).
Burgos-Robles, A., Vidal-Gonzalez, I. & Quirk, G. J. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J. Neurosci. 29, 8474–8482 (2009).
Namburi, P. et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015).
Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19, 1636–1646 (2016).
Senn, V. et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014).
Xu, C. et al. Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell 167, 961–972.e16 (2016).
Marek, R. et al. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat. Neurosci. 21, 384–392 (2018).
Klavir, O., Prigge, M., Sarel, A., Paz, R. & Yizhar, O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat. Neurosci. 20, 836–844 (2017).
Davis, P., Zaki, Y., Maguire, J. & Reijmers, L. G. Cellular and oscillatory substrates of fear extinction learning. Nat. Neurosci. 20, 1624–1633 (2017).
Vervliet, B., Craske, M. G. & Hermans, D. Fear extinction and relapse: state of the art. Annu. Rev. Clin. Psychol. 9, 215–248 (2013).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Sparta, D. R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2011).
Acknowledgements
We thank J. Dunsmoor for comments on the manuscript. A.F.L. was supported by National Institutes of Health (NIH) grant nos. F31 MH111243 and T32 MH106454. S.L.S. was supported by grant no. PD/BD/128076/2016 from the Portuguese Foundation for Science and Technology. C.A.D. was supported by NIH grant no. DP5 OD017908 and New York Stem Cell Science grant no. C-029157. M.R.D. was supported by NIH grant nos. R01 MH102595, R01 MH117426 and R21 EY026446.
Author information
Authors and Affiliations
Contributions
A.F.L., C.A.D., and M.R.D. conceived the project and designed the experiments. A.F.L., S.C.L., and S.L.S. performed the cell reactivation experiments. A.F.L. performed the optogenetic behavioral experiments. A.F.L., E.T.B., C.R.C., F.S., M.J.M., K.P.S., S.C.L., and S.L.S. quantified the cells and analyzed the data. C.A.D. generated the ArcCreERT2 mice. A.F.L. and M.R.D. wrote the manuscript. M.R.D. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Neuroscience thanks Denise Cai and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Alternative reactivation metrics.
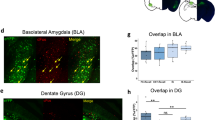
(a, b) From Fig. 1. Extinction and alternative context exposure decreased the (a) percentage of Arc+ cells among eYFP+ cells and (b) percentage of eYFP+ cells among Arc+ cells compared to fear retrieval. No Ext: n = 8 mice; Ext: n = 8 mice; Alt Ctx: n = 6 mice. (c, d) From Fig. 2. During an extinction retrieval test, Ext-Tag mice displayed a (c) trend towards a greater percentage of Arc+ cells among eYFP+ cells and (d) a greater percentage of eYFP+ cells among Arc+ cells compared to Acq-Tag mice. The pattern reversed during the spontaneous recovery test. Acq-Tag/ExtTest: n = 8 mice; Ext-Tag/ExtTest: n = 8 mice; Acq-Tag/SpontRec: n = 8 mice; Ext-Tag/SpontRec: n = 7 mice. Data are means ± s.e.m. See Supplementary Table 1 for statistics. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Supplementary Figure 2 Silencing extinction- and fear acquisition-tagged neurons has no effect during second light ON and OFF epochs.
(a-c) From extinction-tagged neuron silencing experiment (Fig. 3). Silencing extinction-tagged neurons had no effect on freezing behavior during the second light ON and OFF epochs during (a) extinction retrieval test, (b) alternate context test, and (c) spontaneous recovery test. ArcCreERT2(-): n = 9 mice; ArcCreERT2(+): n = 9 mice. (d-f) From fear-tagged neuron silencing experiment (Fig. 4). Silencing fear-tagged neurons had no effect on freezing behavior during the second light ON and OFF epochs during (d) extinction retrieval test, (e) alternate context test, and (f) spontaneous recovery test. ArcCreERT2(-): n = 8 mice; ArcCreERT2(+): n = 7 mice. Data are means ± s.e.m. See Supplementary Table 1 for statistics.
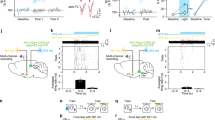
Supplementary Figure 3 Silencing extinction-tagged neurons impairs extinction retrieval in second extinction retrieval test.
(a) Experimental design. Second extinction retrieval test occurred 1 d after the alternate context test. Same animals from Fig. 3. (b) Freezing behavior during the second extinction retrieval test. (c) Silencing extinction-tagged neurons increased freezing in ArcCreERT2(+) mice during the first light ON and light OFF epochs compared to ArcCreERT2(-) mice. ArcCreERT2(-): n = 9 mice; ArcCreERT2(+): n = 9 mice. Data are means ± s.e.m. See Supplementary Table 1 for statistics. * p < 0.05, ** p < 0.01.
Supplementary Figure 4 Stimulating fear acquisition- or extinction-tagged neurons induces c-Fos expression in eYFP+ cells in dorsal DG and CA3.
(a) Representative images of eYFP+ and c-Fos+ immunofluorescence in the DG of Acq-Tag(+) mice presented with light (top) or without light (bottom). White arrowheads denote co-labeled eYFP+/Arc+ cells. (b) Light increased the percentage of c-Fos+ cells among eYFP+ cells in Acq-Tag(+) and Ext-Tag(+) mice in the DG near the fiber optic location. (c) Same as (a), but in CA3. (d) Light increased the percentage of c-Fos+ cells among eYFP+ cells in Acq-Tag(+) and Ext-Tag(+) mice in dorsal CA3. Light: n = 5 mice; No Light: n = 3 mice. Data are means ± s.e.m. See Supplementary Table 1 for statistics. * p < 0.05, ** p < 0.01.
Supplementary Figure 5 Stimulating fear acquisition- or extinction-tagged neurons does not affect anxiety or exploratory behaviors.
(a) OF design. Light ON epoch occurred during minutes 5–10. Same animals from Fig. 5. (b-d) Stimulating fear acquisition- or extinction-tagged neurons did not affect (b) percentage of time in OF center, (c) center distance traveled, or (d) total distance traveled. Light OFF epochs averaged. Acq/Ext-Tag(-): n = 12 mice; Acq-Tag(+): n = 4 mice; Ext-Tag(+): n = 4 mice. Data are means ± s.e.m. See Supplementary Table 1 for statistics.
Supplementary Figure 6 Stimulating extinction-tagged neurons promotes extinction retrieval in extinction retrieval test following spontaneous recovery.
(a) Experimental design. Second extinction retrieval test occurred 1 d after spontaneous recovery test. Light was delivered during minutes 3–6. Same animals from Fig. 5. (b) Freezing behavior during the second extinction retrieval test. (c) Stimulating extinction-tagged neurons reduced freezing during the light ON epoch in Ext-Tag(+) mice compared to Acq/Ext-Tag(-) mice. Light OFF epochs averaged. Acq/Ext-Tag(-): n = 12 mice; Acq-Tag(+): n = 4 mice; Ext-Tag(+): n = 4 mice. Data are means ± s.e.m. See Supplementary Table 1 for statistics. * p < 0.05.
Supplementary information
Rights and permissions
About this article
Cite this article
Lacagnina, A.F., Brockway, E.T., Crovetti, C.R. et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci 22, 753–761 (2019). https://doi.org/10.1038/s41593-019-0361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0361-z
This article is cited by
-
Reactivation of encoding ensembles in the prelimbic cortex supports temporal associations
Neuropsychopharmacology (2024)
-
Fear extinction is impaired in aged rats
GeroScience (2024)
-
Context Processing in Contextual and Cued Fear Extinction
Neuroscience Bulletin (2024)
-
Pharmacological diacylglycerol lipase inhibition impairs contextual fear extinction in mice
Psychopharmacology (2024)
-
Locus coeruleus input-modulated reactivation of dentate gyrus opioid-withdrawal engrams promotes extinction
Neuropsychopharmacology (2023)