Abstract
Motor behaviour is most efficiently controlled by correcting only disturbances that influence task success. It is currently thought that such control is computed within a transcortical feedback pathway. Here we show that, for postural hand control, even the fastest spinal feedback pathway can produce efficient corrective responses, forcing a re-evaluation of how the nervous system derives the control laws that support motor behavior.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Code availability
MATLAB code used for data analyses is available from the corresponding author upon reasonable request.
Data availability
Raw kinematic and EMG data from these experiments are available from the corresponding author upon reasonable request.
Change history
07 March 2019
In the version of this article initially published, the reviewer recognition statement was missing. The error has been corrected in the HTML and PDF versions of the article.
References
Todorov, E. Nat. Neurosci. 7, 907–915 (2004).
Cole, K. J., Gracco, V. L. & Abbs, J. H. Exp. Brain Res. 56, 582–585 (1984).
Scott, S. H. Trends Neurosci. 39, 512–526 (2016).
Cheney, P. D. & Fetz, E. E. J. Physiol. (Lond.) 349, 249–272 (1984).
Evarts, E. V. & Tanji, J. J. Neurophysiol. 39, 1069–1080 (1976).
Omrani, M., Murnaghan, C. D., Pruszynski, J. A. & Scott, S. H. eLife 5, e13141 (2016).
Pruszynski, J. A. et al. Nature 478, 387–390 (2011).
Pruszynski, J. A. & Scott, S. H. Exp. Brain Res. 218, 341–359 (2012).
Vallbo, A. B. Acta Physiol. Scand. 90, 319–336 (1974).
Pierrot-Deseilligny, E. & Burke, D. The Circuitry of the Human Spinal Cord: Spinal and Cortical Mechanisms of Movement. 2nd ed. (Cambridge Univ. Press, Cambridge, UK, 2012).
Mortimer, J. A., Webster, D. D. & Dukich, T. G. Brain Res. 229, 337–351 (1981).
Dufresne, J. R., Soechting, J. F. & Terzuolo, C. A. Brain Res. 193, 67–84 (1980).
Thompson, A. K., Chen, X. Y. & Wolpaw, J. R. J. Neurosci. 29, 5784–5792 (2009).
Schouenborg, J. Action-based sensory encoding in spinal sensorimotor circuits. Brain Res. Rev. 57, 111–117 (2008).
Manning, C. D. & Bawa, P. J. Neurophysiol. 106, 1489–1499 (2011).
Kurtzer, I. L., Pruszynski, J. A. & Scott, S. H. Curr. Biol. 18, 449–453 (2008).
Soechting, J. F. & Lacquaniti, F. J. Neurophysiol 59, 1296–1313 (1988).
Giszter, S. F., McIntyre, J. & Bizzi, E. J. Neurophysiol. 62, 750–767 (1989).
Fukson, O. I., Berkinblit, M. B. & Feldman, A. G. Science 209, 1261–1263 (1980).
Fink, A. J. et al. Nature 509, 43–48 (2014).
Crevecoeur, F. & Scott, S. H. PLoS Comput. Biol. 9, e1003177 (2013).
Crevecoeur, F., Thonnard, J. L., Lefèvre, P.& Scott, S. H. eNeuro 3, e0129-15.2016 https://doi.org/10.1523/ENEURO.0129-15.2016 (2016).
Pruszynski, J. A., Kurtzer, I. & Scott, S. H. J. Neurophysiol. 100, 224–238 (2008).
Weiler, J., Gribble, P. L. & Pruszynski, J. A. J. Neurophysiol. 114, 3242–3254 (2015).
Weiler, J., Saravanamuttu, J., Gribble, P. L. & Pruszynski, J. A. J. Neurophysiol. 116, 2236–2249 (2016).
Yang, L., Michaels, J. A., Pruszynski, J. A. & Scott, S. H. Exp. Brain Res. 211, 231–242 (2011).
Acknowledgements
This work was supported by the Natural Science and Engineering Council of Canada (NSERC Discovery Grants RGPIN-2015-06714 (to J.A.P.) and RGPIN-2018-05458 (to P.L.G.)). J.W. was supported by post-doctoral fellowships from NSERC and the BrainsCAN program at Western University. J.A.P. received a salary award from the Canada Research Chairs Program. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.W. and J.A.P. designed the study, J.W. collected and analysed the data, and J.W., P.L.G. and J.A.P. interpreted the results and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Neuroscience thanks Simon Giszter and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
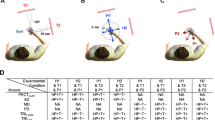
Supplementary Figure 1 Experimental loads
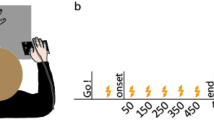
Mechanical torques (loads) applied to the elbow (top) and wrist (bottom) across trial types for each of the seven experiments (a–f). Positive values representflexion loads. i, Participants placed their hand to the home location for 500 ms. ii, A flexion or extension ramping load was applied at the elbow and wrist over 1,500 ms, plateauing at ± 2 Nm and ± 1 Nm at the elbow and wrist, respectively (the pre-load). iii, Participants moved their hand to a central target and stayed at this location for a randomized foreperiod (2,500–3,500 ms). iv, A 20 Nm step-torque in the direction of the pre-load was applied at the elbow and a 1 Nm flexion or extension step-torque was simultaneously applied at the wrist (the perturbation). v, Participants moved the hand back to the target following the perturbation. The load associated with the perturbation remained on for 1,300 ms. vi, The loads were gradually removed over 1,000 ms. Note that the extension pre-load used in the ‘flip’ condition of experiment 7 activates the wrist extensor muscles and that the wrist perturbations across all experiments are colour coded to correspond with the data presented in all other figures.
Supplementary Figure 2 Biceps spinal reflex is tuned to hand displacement
a, Mean rectified biceps EMG activity when the wrist flexor muscles were pre-excited prior to the mechanical perturbation (experiment 2; n = 20). Green and blue traces representwrist perturbations that flexed and extended the wrist, respectively, whereas the red trace represents trials in which no perturbation was applied to the wrist. Data are aligned to perturbation onset. Shading represents1 s.e.m. b,Mean rectified biceps EMG activity in the spinal stretch reflex epoch for the three wrist perturbations (F flexion; N, none; E, extension). Thin grey lines represent individual participants and the thick black lines represent the group mean. The biceps spinal stretch reflex was tuned to the hand’s displacement from the target, and not to the elbow extension, (F(2,38) = 44.47, p < 0.001, η2partial = 0.701; post-hoc trend analysis, linear F(1,19) = 47.21, p < 0.001, η2partial = 0.713; quadratic F(1,19) = 5.43, p = 0.03, η2partial = 0.22).
Supplementary Figure 3 Spinal reflexes are not influenced by volitional intent
a, Mean rectified triceps EMG activity following the mechanical perturbations that flexed the elbow and flexed the wrist (experiment 3; n = 15). Red and blue traces represent ‘counteract’ and ‘do not intervene’ blocks, respectively. Data are aligned to perturbation onset. Shading represents ± 1 s.e.m. b, Goal-dependent activity within the spinal (SR) and long-latency (LL) epochs for trials in which the mechanical perturbation flexed the elbow and flexed the wrist. Error bars represent 95% confidence internals. c, Same format as a, but for trials in which the elbow was flexed and no perturbation was applied to the wrist. d, Same format as b, but for trials in which the elbow was flexed and no perturbation was applied to the wrist. e, Same format as a, but for trials in which the mechanical perturbations flexed the elbow and extended the wrist. f, Same format as b, but for trials in which the mechanical perturbations flexed the elbow and extended the wrist. The magnitude of the triceps spinal stretch reflex was not influenced by the intended action, when the wrist was flexed, not perturbed, or extended (ts(14) all < 1.58, ps all > 0.135, all Cohen d < 0.42). In contrast, the triceps long-latency stretch reflex was influenced by the intended action, and this occurred for all three wrist perturbation conditions (ts(14) all > 4.01, ps all < 0.001, all Cohen d > 1.0: repeated measures ANOVA three-way interaction [epoch (spinal, long-latency) by wrist perturbation (flexed, neutral, extended) by volitional intent (counteract, do not intervene)] for initial omnibus test (F(2,28) = 17.19, p < 0.001, η2partial = 0.55).
Supplementary Figure 4 Determining the source of wrist afferent feedback that tunes the biceps spinal reflex
a, Mean rectified biceps EMG activity when the wrist extensors were pre-excited prior to the mechanical perturbation (experiment 5; n = 20). Green and blue traces represent wrist perturbations that flexed and extended the wrist, respectively. Data is aligned to perturbation onset. Shading represents ± 1 s.e.m. b, Mean rectified biceps EMG activity in the spinal stretch reflex epoch for when the perturbation flexed (F) or extended (E) the wrist. Thin grey lines represent individual participants and the thick black line represents the group mean. The biceps spinal stretch reflex was not influenced by the perturbations applied at the wrist (t(14) = -1.13 p = 0.28, Cohen’s d = 0.29, 95% CI [-0.15 -0.05]).
Supplementary Figure 5 Wrist muscle activity for the upright and flipped orientations
a, Mean rectified wrist extensor EMG activity for perturbations that flexed the elbow and flexed the wrist (Experiment 7: n = 15). Blue and red traces represent the Upright and Flipped arm orientations, respectively. Data aligned to perturbation onset. Shading reflects ± 1 SEM. b, Mean rectified wrist extensor EMG activity in the spinal stretch reflex epoch when the wrist was flexed as a function of the upright and flipped orientations. Thin grey lines represent individual participants, whereas the thick black line represents the group mean. c, Same format as a, but for trials in which the wrist was extended. d, Same format as b, but when the wrist was extended.
Supplementary information
Rights and permissions
About this article
Cite this article
Weiler, J., Gribble, P.L. & Pruszynski, J.A. Spinal stretch reflexes support efficient hand control. Nat Neurosci 22, 529–533 (2019). https://doi.org/10.1038/s41593-019-0336-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0336-0
This article is cited by
-
Cerebellar associative learning underlies skilled reach adaptation
Nature Neuroscience (2023)
-
The capacity of action observation to drag the trainees' motor pattern toward the observed model
Scientific Reports (2023)
-
Epidural electrical stimulation of the cervical dorsal roots restores voluntary upper limb control in paralyzed monkeys
Nature Neuroscience (2022)
-
A neuromuscular model of human locomotion combines spinal reflex circuits with voluntary movements
Scientific Reports (2022)
-
Spinal Cord Circuits: Models and Reality
Neurophysiology (2021)