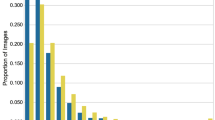
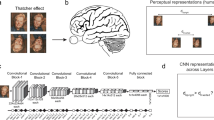
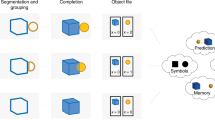
Abstract
Our knowledge of sensory processing has advanced dramatically in the last few decades, but this understanding remains far from complete, especially for stimuli with the large dynamic range and strong temporal and spatial correlations characteristic of natural visual inputs. Here we describe some of the issues that make understanding the encoding of natural images a challenge. We highlight two broad strategies for approaching this problem: a stimulus-oriented framework and a goal-oriented one. Different contexts can call for one framework or the other. Looking forward, recent advances, particularly those based in machine learning, show promise in borrowing key strengths of both frameworks and by doing so illuminating a path to a more comprehensive understanding of the encoding of natural stimuli.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gollisch, T. & Meister, M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron 65, 150–164 (2010).
Schwartz, G. W. & Rieke, F. Nonlinear spatial encoding by retinal ganglion cells: when 1 + 1 ! = 2. J. Gen. Physiol. 138, 283–290 (2011).
Demb, J. B. & Singer, J. H. Functional circuitry of the retina. Annu. Rev. Vis. Sci. 1, 263–289 (2015).
Graham, N. V. Beyond multiple pattern analyzers modeled as linear filters (as classical V1 simple cells): useful additions of the last 25 years. Vision Res. 51, 1397–1430 (2011).
Rieke, F. & Rudd, M. E. The challenges natural images pose for visual adaptation. Neuron 64, 605–616 (2009).
Solomon, S. G. & Kohn, A. Moving sensory adaptation beyond suppressive effects in single neurons. Curr. Biol. 24, R1012–R1022 (2014).
Baddeley, R. et al. Responses of neurons in primary and inferior temporal visual cortices to natural scenes. Proc. Biol. Sci. 264, 1775–1783 (1997).
Creutzfeldt, O. D. & Nothdurft, H. C. Representation of complex visual stimuli in the brain. Naturwissenschaften 65, 307–318 (1978).
Smyth, D., Willmore, B., Baker, G. E., Thompson, I. D. & Tolhurst, D. J. The receptive-field organization of simple cells in primary visual cortex of ferrets under natural scene stimulation. J. Neurosci. 23, 4746–4759 (2003).
Stanley, G. B., Li, F. F. & Dan, Y. Reconstruction of natural scenes from ensemble responses in the lateral geniculate nucleus. J. Neurosci. 19, 8036–8042 (1999).
Vickers, N. J., Christensen, T. A., Baker, T. C. & Hildebrand, J. G. Odour-plume dynamics influence the brain’s olfactory code. Nature 410, 466–470 (2001).
Vinje, W. E. & Gallant, J. L. Sparse coding and decorrelation in primary visual cortex during natural vision. Science 287, 1273–1276 (2000).
Sharpee, T. O. et al. Adaptive filtering enhances information transmission in visual cortex. Nature 439, 936–942 (2006).
Theunissen, F. E. & Elie, J. E. Neural processing of natural sounds. Nat. Rev. Neurosci. 15, 355–366 (2014).
Zwicker, D., Murugan, A. & Brenner, M. P. Receptor arrays optimized for natural odor statistics. Proc. Natl Acad. Sci. USA 113, 5570–5575 (2016).
Carandini, M. et al. Do we know what the early visual system does? J. Neurosci. 25, 10577–10597 (2005).
David, S. V. & Gallant, J. L. Predicting neuronal responses during natural vision. Network 16, 239–260 (2005).
Turner, M. H. & Rieke, F. Synaptic rectification controls nonlinear spatial integration of natural visual inputs. Neuron 90, 1257–1271 (2016).
Heitman, A. et al. Testing pseudo-linear models of responses to natural scenes in primate retina. Preprint at bioRxiv https://doi.org/10.1101/045336 (2016).
Maheswaranathan, N., Kastner, D. B., Baccus, S. A. & Ganguli, S. Inferring hidden structure in multilayered neural circuits. PLoS Comput. Biol. 14, e1006291 (2018).
McIntosh, L. T., Maheswaranathan, N., Nayebi, A., Ganguli, S. & Baccus, S. A. Deep learning models of the retinal response to natural scenes. Adv. Neural Inf. Process. Syst. 29, 1369–1377 (2016).
Felsen, G., Touryan, J., Han, F. & Dan, Y. Cortical sensitivity to visual features in natural scenes. PLoS Biol. 3, e342 (2005).
Rust, N. C., Schwartz, O., Movshon, J. A. & Simoncelli, E. P. Spatiotemporal elements of macaque V1 receptive fields. Neuron 46, 945–956 (2005).
Eickenberg, M., Rowekamp, R. J., Kouh, M. & Sharpee, T. O. Characterizing responses of translation-invariant neurons to natural stimuli: maximally informative invariant dimensions. Neural Comput. 24, 2384–2421 (2012).
Vintch, B., Movshon, J. A. & Simoncelli, E. P. A convolutional subunit model for neuronal responses in macaque V1. J. Neurosci. 35, 14829–14841 (2015).
Rowekamp, R. J. & Sharpee, T. O. Cross-orientation suppression in visual area V2. Nat. Commun. 8, 15739 (2017).
Pagan, M., Simoncelli, E. P. & Rust, N. C. Neural quadratic discriminant analysis: nonlinear decoding with V1-like computation. Neural Comput. 28, 1–29 (2016).
Hyvärinen, A. Statistical models of natural images and cortical visual representation. Top. Cogn. Sci. 2, 251–264 (2010).
Lewicki, M. S., Olshausen, B. A., Surlykke, A. & Moss, C. F. Scene analysis in the natural environment. Front. Psychol. 5, 199 (2014).
Simoncelli, E. P. & Olshausen, B. A. Natural image statistics and neural representation. Annu. Rev. Neurosci. 24, 1193–1216 (2001).
Zhaoping, L. Theoretical understanding of the early visual processes by data compression and data selection. Network 17, 301–334 (2006).
Coen-Cagli, R., Dayan, P. & Schwartz, O. Cortical surround interactions and perceptual salience via natural scene statistics. PLoS Comput. Biol. 8, e1002405 (2012).
Frazor, R. A. & Geisler, W. S. Local luminance and contrast in natural images. Vision Res. 46, 1585–1598 (2006).
Karklin, Y. & Lewicki, M. S. A hierarchical Bayesian model for learning nonlinear statistical regularities in nonstationary natural signals. Neural Comput. 17, 397–423 (2005).
Parra, L., Spence, C. & Sajda, P. Higher-order statistical properties arising from the non-stationarity of natural signals. Adv. Neural Inf. Process. Syst. 14, 786–792 (2001).
Ruderman, D. L. & Bialek, W. Statistics of natural images: Scaling in the woods. Phys. Rev. Lett. 73, 814–817 (1994).
Portilla, J. & Simoncelli, E. P. Parametric texture model based on joint statistics of complex wavelet coefficients. Int. J. Comput. Vis. 40, 49–70 (2000).
Gatys, L. A., Ecker, A. S. & Bethge, M. Texture synthesis using convolutional neural networks. Adv. Neural Inf. Process. Syst. 28, 262–270 (2015).
Karras, T., Aila, T., Laine, S. & Lehtinen, J. Progressive Growing of GANs for Improved Quality, Stability, and Variation. Preprint at arXiv https://arxiv.org/abs/1710.10196 (2018).
Freeman, J., Ziemba, C. M., Heeger, D. J., Simoncelli, E. P. & Movshon, J. A. A functional and perceptual signature of the second visual area in primates. Nat. Neurosci. 16, 974–981 (2013).
Okazawa, G., Tajima, S. & Komatsu, H. Image statistics underlying natural texture selectivity of neurons in macaque V4. Proc. Natl Acad. Sci. USA 112, E351–E360 (2015).
Rust, N. C. & Dicarlo, J. J. Selectivity and tolerance (“invariance”) both increase as visual information propagates from cortical area V4 to IT. J. Neurosci. 30, 12978–12995 (2010).
Atick, J. J. & Redlich, A. N. What does the retina know about natural scenes? Neural Comput. 4, 196–210 (1992).
Srinivasan, M. V., Laughlin, S. B. & Dubs, A. Predictive coding: a fresh view of inhibition in the retina. Proc. R. Soc. Lond. B Biol. Sci. 216, 427–459 (1982).
Marr, D. & Hildreth, E. Theory of edge detection. Proc. R. Soc. Lond. B Biol. Sci. 207, 187–217 (1980).
Zhaoping, L. Understanding Vision: Theory, Models, and Data. (Oxford University Press, Oxford, UK, (2014).
Barlow, H.B. Possible principles underlying the transformations of sensory messages. in Sensory Communication (ed. W.A. Rosenblith) 217–234 (Wiley, Oxford, UK, 1961).
Attneave, F. Some informational aspects of visual perception. Psychol. Rev. 61, 183–193 (1954).
Shannon, C. E. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948).
Field, D. J. What is the goal of sensory coding? Neural Comput. 6, 559–601 (1994).
Bhandawat, V., Olsen, S. R., Gouwens, N. W., Schlief, M. L. & Wilson, R. I. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat. Neurosci. 10, 1474–1482 (2007).
Laughlin, S. A simple coding procedure enhances a neuron’s information capacity. Z. Naturforsch., C, Biosci. 36, 910–912 (1981).
Brinkman, B. A. W., Weber, A. I., Rieke, F. & Shea-Brown, E. How do efficient coding strategies depend on origins of noise in neural circuits? PLoS Comput. Biol. 12, e1005150 (2016).
Gjorgjieva, J., Sompolinsky, H. & Meister, M. Benefits of pathway splitting in sensory coding. J. Neurosci. 34, 12127–12144 (2014).
Kastner, D. B., Baccus, S. A. & Sharpee, T. O. Critical and maximally informative encoding between neural populations in the retina. Proc. Natl Acad. Sci. USA 112, 2533–2538 (2015).
Field, D. J. Relations between the statistics of natural images and the response properties of cortical cells. J. Opt. Soc. Am. A 4, 2379–2394 (1987).
Ruderman, D. L. Origins of scaling in natural images. Vision Res. 37, 3385–3398 (1997).
Dan, Y., Atick, J. J. & Reid, R. C. Efficient coding of natural scenes in the lateral geniculate nucleus: experimental test of a computational theory. J. Neurosci. 16, 3351–3362 (1996).
Franke, K. et al. Inhibition decorrelates visual feature representation in the inner retina. Nature 542, 439–444 (2017).
Pitkow, X. & Meister, M. Decorrelation and efficient coding by retinal ganglion cells. Nat. Neurosci. 15, 628–635 (2012).
Vincent, B. T. & Baddeley, R. J. Synaptic energy efficiency in retinal processing. Vision Res. 43, 1283–1290 (2003).
Atick, J. J. Could information theory provide an ecological theory of sensory processing? Network 22, 4–44 (2011).
Li, Z. & Atick, J. J. Efficient stereo coding in the multiscale representation. Network 5, 157–174 (1994).
Kuang, X., Poletti, M., Victor, J. D. & Rucci, M. Temporal encoding of spatial information during active visual fixation. Curr. Biol. 22, 510–514 (2012).
Segal, I. Y. et al. Decorrelation of retinal response to natural scenes by fixational eye movements. Proc. Natl Acad. Sci. USA 112, 3110–3115 (2015).
Boi, M., Poletti, M., Victor, J. D. & Rucci, M. Consequences of the oculomotor cycle for the dynamics of perception. Curr. Biol. 27, 1268–1277 (2017).
Hyvärinen, A., Hurri, J. & Hoyer, P. O. Natural Image Statistics: a Probabilistic Approach to Early Computational Vision. (Springer-Verlag, London, UK, 2009).
Bell, A. J. & Sejnowski, T. J. The “independent components” of natural scenes are edge filters. Vision Res. 37, 3327–3338 (1997).
Olshausen, B. A. & Field, D. J. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature 381, 607–609 (1996).
Rehn, M. & Sommer, F. T. A network that uses few active neurones to code visual input predicts the diverse shapes of cortical receptive fields. J. Comput. Neurosci. 22, 135–146 (2007).
Eichhorn, J., Sinz, F. & Bethge, M. Natural image coding in V1: how much use is orientation selectivity? PLoS Comput. Biol. 5, e1000336 (2009).
Golden, J. R., Vilankar, K. P., Wu, M. C. K. & Field, D. J. Conjectures regarding the nonlinear geometry of visual neurons. Vision Res. 120, 74–92 (2016).
Schwartz, O. & Simoncelli, E. P. Natural signal statistics and sensory gain control. Nat. Neurosci. 4, 819–825 (2001).
Karklin, Y. & Lewicki, M. S. Emergence of complex cell properties by learning to generalize in natural scenes. Nature 457, 83–86 (2009).
Lochmann, T., Ernst, U. A. & Denève, S. Perceptual inference predicts contextual modulations of sensory responses. J. Neurosci. 32, 4179–4195 (2012).
Rao, R. P. N. & Ballard, D. H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87 (1999).
Spratling, M. W. Predictive coding as a model of response properties in cortical area V1. J. Neurosci. 30, 3531–3543 (2010).
Zhu, M. & Rozell, C. J. Visual nonclassical receptive field effects emerge from sparse coding in a dynamical system. PLoS Comput. Biol. 9, e1003191 (2013).
Berkes, P. & Wiskott, L. Slow feature analysis yields a rich repertoire of complex cell properties. J. Vis. 5, 579–602 (2005).
Cadieu, C. F. & Olshausen, B. A. Learning intermediate-level representations of form and motion from natural movies. Neural Comput. 24, 827–866 (2012).
Coen-Cagli, R. & Schwartz, O. The impact on midlevel vision of statistically optimal divisive normalization in V1. J. Vis. 13, 1–20 (2013).
Hosoya, H. & Hyvärinen, A. A hierarchical statistical model of natural images explains tuning properties in V2. J. Neurosci. 35, 10412–10428 (2015).
Lee, H., Ekanadham, C. & Ng, A. Y. Sparse deep belief net model for visual area V2. Adv. Neural Inf. Process. Syst. 20, 873–880 (2008).
Shan, H. & Cottrell, G. Efficient visual coding: from retina to V2. Preprint at arXiv https://arxiv.org/abs/1312.6077. (2013).
Dayan, P., Sahani, M. & Deback, G. Adaptation and Unsupervised Learning. Adv. Neural Inf. Process. Syst. 15, 237–244 (2003).
Hinton, G. E. & Ghahramani, Z. Generative models for discovering sparse distributed representations. Phil. Trans. R. Soc. Lond. B 352, 1177–1190 (1997).
Wainwright, M. J. & Simoncelli, E. P. Scale mixtures of Gaussians and the statistics of natural images. Adv. Neural Inf. Process. Syst. 12, 855–861 (2000).
Coen-Cagli, R., Kohn, A. & Schwartz, O. Flexible gating of contextual influences in natural vision. Nat. Neurosci. 18, 1648–1655 (2015).
Li, Z. Contextual influences in V1 as a basis for pop out and asymmetry in visual search. Proc. Natl Acad. Sci. USA 96, 10530–10535 (1999).
Lettvin, J. Y., Maturana, H. R., McCulloch, W. S. & Pitts, W. H. What the frog’s eye tells the frog’s brain. Proc. IRE 47, 1940–1951 (1959).
Masland, R. H. & Martin, P. R. The unsolved mystery of vision. Curr. Biol. 17, R577–R582 (2007).
Nath, A. & Schwartz, G. W. Cardinal orientation selectivity is represented by two distinct ganglion cell types in mouse retina. J. Neurosci. 36, 3208–3221 (2016).
Schwartz, G., Harris, R., Shrom, D. & Berry, M. J. II Detection and prediction of periodic patterns by the retina. Nat. Neurosci. 10, 552–554 (2007).
Krishnamoorthy, V., Weick, M. & Gollisch, T. Sensitivity to image recurrence across eye-movement-like image transitions through local serial inhibition in the retina. eLife 6, e22431 (2017).
Franke, F. et al. Structures of neural correlation and how they favor coding. Neuron 89, 409–422 (2016).
Zylberberg, J., Cafaro, J., Turner, M. H., Shea-Brown, E. & Rieke, F. Direction-selective circuits shape noise to ensure a precise population code. Neuron 89, 369–383 (2016).
Rodieck, R. W. The First Steps in Seeing. (Oxford Press, Oxford, UK, 1998).
Hecht, S. & Verrijp, C. D. Intermittent stimulation by light III. The relation between intensity and critical fusion frequency for different retinal locations. J. Gen. Physiol. 17, 251–268 (1933).
Sinha, R. et al. Cellular and circuit mechanisms shaping the perceptual properties of the primate fovea. Cell 168, 413–426.e12 (2017).
Solomon, S. G., Martin, P. R., White, A. J. R., Rüttiger, L. & Lee, B. B. Modulation sensitivity of ganglion cells in peripheral retina of macaque. Vision Res. 42, 2893–2898 (2002).
Oyster, C. W. & Barlow, H. B. Direction-selective units in rabbit retina: distribution of preferred directions. Science 155, 841–842 (1967).
Hughes, S. et al. Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye (Lond.) 30, 247–254 (2016).
Hausen, K. & Egelhaaf, M. in Facets of Vision (eds. Stavenga, D.G. & Hardie, R.C.) 391–424 (Springer, London, UK, 1989).
O’Carroll, D. C., Bidwell, N. J., Laughlin, S. B. & Warrant, E. J. Insect motion detectors matched to visual ecology. Nature 382, 63–66 (1996).
Krapp, H. G. & Hengstenberg, R. Estimation of self-motion by optic flow processing in single visual interneurons. Nature 384, 463–466 (1996).
Longden, K. D., Wicklein, M., Hardcastle, B. J., Huston, S. J. & Krapp, H. G. Spike burst coding of translatory optic flow and depth from motion in the fly visual system. Curr. Biol. 27, 3225–3236.e3 (2017).
Franz, M. O. & Krapp, H. G. Wide-field, motion-sensitive neurons and matched filters for optic flow fields. Biol. Cybern. 83, 185–197 (2000).
Kohn, J. R., Heath, S. L. & Behnia, R. Eyes matched to the prize: the state of matched filters in insect visual circuits. Front. Neural Circuits 12, 26 (2018).
Sabbah, S. et al. A retinal code for motion along the gravitational and body axes. Nature 546, 492–497 (2017).
Gauvain, G. & Murphy, G. J. Projection-specific characteristics of retinal input to the brain. J. Neurosci. 35, 6575–6583 (2015).
Burge, J. & Jaini, P. Accuracy maximization analysis for sensory-perceptual tasks: computational improvements, filter robustness, and coding advantages for scaled additive noise. PLoS Comput. Biol. 13, e1005281 (2017).
Geisler, W. S., Najemnik, J. & Ing, A. D. Optimal stimulus encoders for natural tasks. J. Vis. 9, 1–16 (2009).
Krizhevsky, A., Sutskever, I. & Hinton, G. E. ImageNet classification with deep convolutional neural networks. Adv. Neural Inf. Process. Syst. 25, 1–9 (2012).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Maheswaranathan, N. et al. Deep learning models reveal internal structure and diverse computations in the retina under natural scenes. Preprint at bioRxiv https://doi.org/10.1101/340943 (2018).
Kriegeskorte, N. Deep neural networks: a new framework for modeling biological vision and brain information processing. Annu. Rev. Vis. Sci. 1, 417–446 (2015).
Yamins, D. L. K. & DiCarlo, J. J. Using goal-driven deep learning models to understand sensory cortex. Nat. Neurosci. 19, 356–365 (2016).
Cadena, S. A. et al. Deep convolutional models improve predictions of macaque V1 responses to natural images. Preprint at bioRxiv https://doi.org/10.1101/201764 (2017).
Cichy, R. M., Khosla, A., Pantazis, D., Torralba, A. & Oliva, A. Comparison of deep neural networks to spatio-temporal cortical dynamics of human visual object recognition reveals hierarchical correspondence. Sci. Rep. 6, 27755 (2016).
Pospisil, D., Pasupathy, A. & Bair, W. Comparing the brain’s representation of shape to that of a deep convolutional neural network. Proc. 9th EAI Int. Conf. Bio-inspired Inf. Commun. Technol. (formerly BIONETICS) 516–523 (2016).
Young, M. P. & Yamane, S. Sparse population coding of faces in the inferotemporal cortex. Science 256, 1327–1331 (1992).
Fukushima, K. Neocognitron: a self organizing neural network model for a mechanism of pattern recognition unaffected by shift in position. Biol. Cybern. 36, 193–202 (1980).
Riesenhuber, M. & Poggio, T. Hierarchical models of object recognition in cortex. Nat. Neurosci. 2, 1019–1025 (1999).
Razavian, A. S., Azizpour, H., Sullivan, J. & Carlsson, S. CNN features off-the-shelf: an astounding baseline for recognition. IEEE Conf. Comput. Vis. Pattern Recog. (CVPR) Workshops 806–813 (2014).
Szegedy, C. et al. Intriguing properties of neural networks. Preprint at arXiv https://arxiv.org/abs/1312.6199 (2014).
Ullman, S., Assif, L., Fetaya, E. & Harari, D. Atoms of recognition in human and computer vision. P roc. Natl Acad. Sci. USA 113, 2744–2749 (2016).
Goodfellow, I.J., Shlens, J. & Szegedy, C. Explaining and harnessing adversarial examples. Preprint at arXiv https://arxiv.org/abs/1412.6572 (2015).
Nayebi, A. & Ganguli, S. Biologically inspired protection of deep networks from adversarial attacks Preprint at arXiv https://arxiv.org/abs/1703.09202v1 (2017).
Brendel, W. & Bethge, M. Comment on ‘Biologically inspired protection of deep networks from adversarial attacks’. Preprint at arXiv https://arxiv.org/abs/1704.01547 (2017).
Nishimoto, S. & Gallant, J. L. A three-dimensional spatiotemporal receptive field model explains responses of area MT neurons to naturalistic movies. J. Neurosci. 31, 14551–14564 (2011).
Berardino, A., Ballé, J., Laparra, V. & Simoncelli, E.P. Eigen-distortions of hierarchical representations. Preprint at arXiv https://arxiv.org/abs/1710.02266v3 (2017).
Han, S. & Vasconcelos, N. Object recognition with hierarchical discriminant saliency networks. Front. Comput. Neurosci. 8, 109 (2014).
Ren, M., Liao, R., Urtasun, R., Sinz, F. H. & Zemel, R. S. Normalizing the normalizers: comparing and extending network normalization schemes. Preprint at arXiv https://arxiv.org/abs/1611.04520 (2017).
Sanchez Giraldo, L.G., Schwartz, O. Integrating flexible normalization into mid-level representations of deep convolutional neural networks. Preprint at arXiv https://arxiv.org/abs/1806.01823 (2018).
Spoerer, C. J., McClure, P. & Kriegeskorte, N. Recurrent convolutional neural networks: A better model of biological object recognition. Front. Psychol. 8, 1551 (2017).
Shwartz-Ziv, R. & Tishby, N. Opening the black box of deep neural networks via information. Preprint at arXiv https://arxiv.org/abs/1703.00810v3 (2017).
Chalk, M., Marre, O. & Tkačik, G. Toward a unified theory of efficient, predictive, and sparse coding. Proc. Natl Acad. Sci. USA 115, 186–191 (2018).
Sederberg, A. J., MacLean, J. N. & Palmer, S. E. Learning to make external sensory stimulus predictions using internal correlations in populations of neurons. Proc. Natl Acad. Sci. USA 115, 1105–1110 (2018).
Kuleshov, V. & Ermon, S. Deep hybrid models: bridging discriminative and generative approaches. Uncertainty in AI http://auai.org/uai2017/proceedings/papers/297.pdf (2017).
Park, I. M. & Pillow, J. W. Bayesian efficient coding. Preprint at bioRxiv https://doi.org/10.1101/178418 (2017).
Ballé, J., Laparra, V. & Simoncelli, E.P. End-to-end optimized image compression. Preprint at arXiv https://arxiv.org/abs/1611.01704 (2017).
Hirayama, J., Hyvärinen, A. & Kawanabe, M. SPLICE: fully tractable hierarchical extension of ICA with pooling. Proc. Mach. Learn. Res. 70, 1491–1500 (2017).
Scholte, H. S., Losch, M. M., Ramakrishnan, K., de Haan, E. H. F. & Bohte, S. M. Visual pathways from the perspective of cost functions and multi-task deep neural networks. Cortex 98, 249–261 (2018).
Kell, A. J. E., Yamins, D. L. K., Shook, E. N., Norman-Haignere, S. V. & McDermott, J. H. A task-optimized neural network replicates human auditory behavior, predicts brain responses, and reveals a cortical processing hierarchy. Neuron 98, 630–644.e16 (2018).
Zhuang, C. D. Y. Using multiple optimization tasks to improve deep neural network models of higher ventral cortex. J.Vis. 18, 905 (2018).
Van Der Linde, I., Rajashekar, U., Bovik, A. C. & Cormack, L. K. DOVES: a database of visual eye movements. Spat. Vis. 22, 161–177 (2009).
Rucci, M. & Victor, J. D. The unsteady eye: an information-processing stage, not a bug. Trends Neurosci. 38, 195–206 (2015).
Thomson, M. G. Visual coding and the phase structure of natural scenes. Network 10, 123–132 (1999).
Acknowledgements
We thank H. Krapp, D. Pospisil, and J. Shlens for helpful feedback on an earlier version of this review. H. Krapp very generously provided the data and schematic shown in Fig. 4a,b. This work was supported by NIH grants F31-EY026288 (to M.H.T.), EY028542 (to F.R.), and a National Science Foundation Grant 1715475 (to O.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turner, M.H., Sanchez Giraldo, L.G., Schwartz, O. et al. Stimulus- and goal-oriented frameworks for understanding natural vision. Nat Neurosci 22, 15–24 (2019). https://doi.org/10.1038/s41593-018-0284-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0284-0
This article is cited by
-
A large-scale fMRI dataset for the visual processing of naturalistic scenes
Scientific Data (2023)
-
Neuro-inspired optical sensor array for high-accuracy static image recognition and dynamic trace extraction
Nature Communications (2023)
-
Naturalistic Scene Modelling: Deep Learning with Insights from Biology
Journal of Signal Processing Systems (2023)
-
In-sensor image memorization and encoding via optical neurons for bio-stimulus domain reduction toward visual cognitive processing
Nature Communications (2022)
-
Understanding the retinal basis of vision across species
Nature Reviews Neuroscience (2020)