Abstract
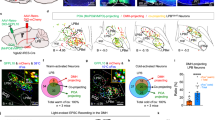
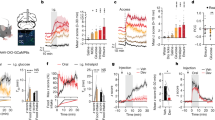
Bombesin-like receptor 3 (BRS3) is an orphan G-protein-coupled receptor that regulates energy homeostasis and heart rate. We report that acute activation of Brs3-expressing neurons in the dorsomedial hypothalamus (DMHBrs3) increased body temperature (Tb), brown adipose tissue temperature, energy expenditure, heart rate, and blood pressure, with no effect on food intake or physical activity. Conversely, activation of Brs3 neurons in the paraventricular nucleus of the hypothalamus had no effect on Tb or energy expenditure, but suppressed food intake. Inhibition of DMHBrs3 neurons decreased Tb and energy expenditure, suggesting a necessary role in Tb regulation. We found that the preoptic area provides major input (excitatory and inhibitory) to DMHBrs3 neurons. Optogenetic stimulation of DMHBrs3 projections to the raphe pallidus increased Tb. Thus, DMHBrs3→raphe pallidus neurons regulate Tb, energy expenditure, and heart rate, and Brs3 neurons in the paraventricular nucleus of the hypothalamus regulate food intake. Brs3 expression is a useful marker for delineating energy metabolism regulatory circuitry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors on reasonable request.
References
Zhang, L. et al. Anatomical characterization of bombesin receptor subtype-3 mRNA expression in the rodent central nervous system. J. Comp. Neurol. 521, 1020–1039 (2013).
Jensen, R. T., Battey, J. F., Spindel, E. R. & Benya, R. V. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 60, 1–42 (2008).
Weber, H. C., Hampton, L. L., Jensen, R. T. & Battey, J. F. Structure and chromosomal localization of the mouse bombesin receptor subtype 3 gene. Gene 211, 125–131 (1998).
Xiao, C. & Reitman, M. L. Bombesin-like receptor 3: physiology of a functional orphan. Trends. Endocrinol. Metab. 27, 603–605 (2016).
Mo, C. et al. Characterization of NMB, GRP and their receptors (BRS3, NMBR and GRPR) in chickens. J. Mol. Endocrinol. 59, 61–79 (2017).
Zhang, Y. et al. Receptor-specific crosstalk between prostanoid E receptor 3 and bombesin receptor subtype 3. FASEB J. 32, 3184–3192 (2018).
Ohki-Hamazaki, H. et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 390, 165–169 (1997).
Ladenheim, E. E. et al. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology 149, 971–978 (2008).
Brommage, R. et al. High-throughput screening of mouse knockout lines identifies true lean and obese phenotypes. Obesity. (Silver. Spring). 16, 2362–2367 (2008).
Lateef, D. M. et al. Bombesin-like receptor 3 regulates blood pressure and heart rate via a central sympathetic mechanism. Am. J. Physiol. Heart Circ. Physiol. 310, H891–H898 (2016).
Lateef, D. M., Abreu-Vieira, G., Xiao, C. & Reitman, M. L. Regulation of body temperature and brown adipose tissue thermogenesis by bombesin receptor subtype-3. Am. J. Physiol. Endocrinol. Metab. 306, E681–E687 (2014).
Guan, X. M. et al. Regulation of energy homeostasis by bombesin receptor subtype-3: selective receptor agonists for the treatment of obesity. Cell. Metab. 11, 101–112 (2010).
Guan, X. M. et al. Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J. Pharmacol. Exp. Ther. 336, 356–364 (2011).
Reitman, M. L. et al. Pharmacokinetics and pharmacodynamics of MK-5046, a bombesin receptor subtype-3 (BRS-3) agonist, in healthy patients. J. Clin. Pharmacol. 52, 1306–1316 (2012).
Nio, Y. et al. A selective bombesin receptor subtype 3 agonist promotes weight loss in male diet-induced-obese rats with circadian rhythm change. Endocrinology 158, 1298–1313 (2017).
Xiao, C. et al. Bombesin-like receptor 3 (Brs3) expression in glutamatergic, but not GABAergic, neurons is required for regulation of energy metabolism. Mol. Metab. 6, 1540–1550 (2017).
Liu, J. et al. Molecular basis of the pharmacological difference between rat and human bombesin receptor subtype-3 (BRS-3). Biochemistry 41, 8954–8960 (2002).
Tan, C. L. et al. Warm-sensitive neurons that control body temperature. Cell 167, 47–59.e15 (2016).
Song, K. et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353, 1393–1398 (2016).
Boulant, J. A. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 31 (Suppl 5), S157–S161 (2000).
Zhao, Z. D. et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 114, 2042–2047 (2017).
Morrison, S. F. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci. 196, 14–24 (2016).
Nakamura, K. & Morrison, S. F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. (Lond.) 589, 3641–3658 (2011).
Cano, G. et al. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 460, 303–326 (2003).
Jeong, J. H. et al. Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Mol. Metab. 4, 483–492 (2015).
Rezai-Zadeh, K. et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 3, 681–693 (2014).
Garfield, A. S. et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 19, 1628–1635 (2016).
Kataoka, N., Hioki, H., Kaneko, T. & Nakamura, K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell. Metab. 20, 346–358 (2014).
Cao, W. H. & Morrison, S. F. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology 51, 426–437 (2006).
Dimicco, J. A. & Zaretsky, D. V. The dorsomedial hypothalamus: a new player in thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R47–R63 (2007).
Zhang, Y. et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 31, 1873–1884 (2011).
Machado, N. L. S. et al. A glutamatergic hypothalamomedullary circuit mediates thermogenesis, but not heat conservation, during stress-induced hyperthermia. Curr. Biol. 28, 2291–2301.e5 (2018).
Allison, M. B. et al. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol. Metab. 4, 299–309 (2015).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Dampney, R. A. Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R429–R443 (2015).
Viollet, C. et al. Somatostatin-IRES-Cre mice: between knockout and wild-type? Front. Endocrinol. (Lausanne) 8, 131 (2017).
Shah, B. P. et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl. Acad. Sci. USA 111, 13193–13198 (2014).
An, J. J., Liao, G. Y., Kinney, C. E., Sahibzada, N. & Xu, B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell. Metab. 22, 175–188 (2015).
Pei, H., Sutton, A. K., Burnett, K. H., Fuller, P. M. & Olson, D. P. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol. Metab. 3, 209–215 (2014).
Sutton, A. K. et al. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J. Neurosci. 34, 15306–15318 (2014).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Nguyen, N. L. et al. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R132–R145 (2017).
Simonds, S. E. et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 159, 1404–1416 (2014).
Thompson, R. H. & Swanson, L. W. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res. Brain Res. Rev. 27, 89–118 (1998).
Fontes, M. A. P. et al. Asymmetric sympathetic output: the dorsomedial hypothalamus as a potential link between emotional stress and cardiac arrhythmias. Auton. Neurosci. 207, 22–27 (2017).
Nakamura, K. Neural circuit for psychological stress-induced hyperthermia. Temperature (Austin) 2, 352–361 (2015).
Nakamura, K. & Morrison, S. F. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R127–R136 (2007).
Morrison, S. F. Central control of body temperature. F1000Res. 5, 880 (2016). F1000 Faculty Rev-.
Cao, W. H., Fan, W. & Morrison, S. F. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126, 229–240 (2004).
Dimitrov, E. L., Kim, Y. Y. & Usdin, T. B. Regulation of hypothalamic signaling by tuberoinfundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation. J. Neurosci. 31, 18166–18179 (2011).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Peron, S. P., Freeman, J., Iyer, V., Guo, C. & Svoboda, K. A cellular resolution map of barrel cortex activity during tactile behavior. Neuron 86, 783–799 (2015).
Szymczak-Workman, A. L., Vignali, K. M. & Vignali, D. A. Generation of 2A-linked multicistronic cassettes by recombinant PCR. Cold Spring Harb. Protoc. 2012, 251–254 (2012).
Ravussin, Y., Gutman, R., LeDuc, C. A. & Leibel, R. L. Estimating energy expenditure in mice using an energy balance technique. Int. J. Obes. (Lond). 37, 399–403 (2013).
Sebhat, I. K. et al. Discovery of MK-5046, a potent, selective bombesin receptor subtype-3 agonist for the treatment of obesity. ACS Med. Chem. Lett. 2, 43–47 (2010).
Graves, J. A. Did sex chromosome turnover promote divergence of the major mammal groups?: De novo sex chromosomes and drastic rearrangements may have posed reproductive barriers between monotremes, marsupials and placental mammals. Bioessays 38, 734–743 (2016).
Qi, J. et al. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. 19, 725–733 (2016).
Stratigopoulos, G. et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J. Clin. Invest. 126, 1897–1910 (2016).
Fox, D. A. et al. Gestational lead exposure selectively decreases retinal dopamine amacrine cells and dopamine content in adult mice. Toxicol. Appl. Pharmacol. 256, 258–267 (2011).
Geerling, J. C., Yokota, S., Rukhadze, I., Roe, D. & Chamberlin, N. L. Kölliker-Fuse GABAergic and glutamatergic neurons project to distinct targets. J. Comp. Neurol. 525, 1844–1860 (2017).
Wu, Z., Autry, A. E., Bergan, J. F., Watabe-Uchida, M. & Dulac, C. G. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330 (2014).
Saunders, A., Johnson, C. A. & Sabatini, B. L. Novel recombinant adeno-associated viruses for Cre activated and inactivated transgene expression in neurons. Front. Neural Circuits 6, 47 (2012).
Rudaya, A. Y., Steiner, A. A., Robbins, J. R., Dragic, A. S. & Romanovsky, A. A. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1244–R1252 (2005).
Lee, D. L., Webb, R. C. & Brands, M. W. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1394–R1398 (2004).
Lute, B. et al. Biphasic effect of melanocortin agonists on metabolic rate and body temperature. Cell. Metab. 20, 333–345 (2014).
Vianna, D. M. L. & Carrive, P. Stress-induced hyperthermia is not mediated by brown adipose tissue in mice. J. Therm. Biol. 37, 125–129 (2012).
Gachkar, S. et al. 3-Iodothyronamine induces tail vasodilation through central action in male mice. Endocrinology 158, 1977–1984 (2017).
Kim, S. M. et al. Salt sensitivity of blood pressure in NKCC1-deficient mice. Am. J. Physiol. Renal. Physiol. 295, F1230–F1238 (2008).
Stornetta, R. L., Inglis, M. A., Viar, K. E. & Guyenet, P. G. Afferent and efferent connections of C1 cells with spinal cord or hypothalamic projections in mice. Brain. Struct. Funct. 221, 4027–4044 (2016).
Betley, J. N., Cao, Z. F., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates. Compact 3rd edn, (Elsevier Academic Press, Cambridge, MA, USA, 2008).
Acknowledgements
We thank A. Kravitz for input throughout the project and critical reading of the manuscript, A. Franks for assistance with animal husbandry, Y. Huang and Y. Ma for assistance with surgeries, and A. Noguchi and D. Springer of the NHLBI Murine Phenotyping Core for the cardiovascular telemetry implantation surgeries. S. Sternson provided the AAV-ChR plasmid construct and R. Neve (Massachusetts General Hospital, Boston, MA), provided HSVs. MK-5046 was generously donated by Merck. Rabies virus was obtained from the GT3 Core Facility of the Salk Institute, which was funded by NIH-NCI CCSG: P30 014195 and NINDS R24 Core Grant and funding from NEI. This research was supported by the Intramural Research Program (DK075057, DK075062, and DK075063 to M.L.R.; DK07002 to M.J.K.) of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
Author information
Authors and Affiliations
Contributions
R.A.P. and M.L.R. conceived and designed the study with input from M.J.K. and O.G. R.A.P. performed and analyzed the experiments. R.A.P., S.H.Z., and A.S. performed chemogenetic experiments and immunohistochemistry and counted cells. R.A.P., S.H.Z., and B.K.T. performed optogenetic and anterograde tracing experiments. C.X. generated Brs3-Cre mice and performed western blots and qRT-PCR experiments. O.G. performed indirect calorimetry experiments. V.S. and R.A.P. performed IR experiments. C.L. performed electrophysiology experiments. R.A.P. wrote the manuscript with input from M.L.R. and all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Piñol, R.A., Zahler, S.H., Li, C. et al. Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat Neurosci 21, 1530–1540 (2018). https://doi.org/10.1038/s41593-018-0249-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0249-3
This article is cited by
-
Lateral Habenula Neurons Signal Cold Aversion and Participate in Cold Aversion
Neurochemical Research (2024)
-
A sex-specific thermogenic neurocircuit induced by predator smell recruiting cholecystokinin neurons in the dorsomedial hypothalamus
Nature Communications (2023)
-
Natural product P57 induces hypothermia through targeting pyridoxal kinase
Nature Communications (2023)
-
A parabrachial-hypothalamic parallel circuit governs cold defense in mice
Nature Communications (2023)
-
Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding
Nature Metabolism (2022)