Abstract
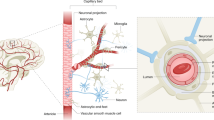
Adequate supply of blood and structural and functional integrity of blood vessels are key to normal brain functioning. On the other hand, cerebral blood flow shortfalls and blood–brain barrier dysfunction are early findings in neurodegenerative disorders in humans and animal models. Here we first examine molecular definition of cerebral blood vessels, as well as pathways regulating cerebral blood flow and blood–brain barrier integrity. Then we examine the role of cerebral blood flow and blood–brain barrier in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and multiple sclerosis. We focus on Alzheimer’s disease as a platform of our analysis because more is known about neurovascular dysfunction in this disease than in other neurodegenerative disorders. Finally, we propose a hypothetical model of Alzheimer’s disease biomarkers to include brain vasculature as a factor contributing to the disease onset and progression, and we suggest a common pathway linking brain vascular contributions to neurodegeneration in multiple neurodegenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Iadecola, C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017).
Kisler, K., Nelson, A. R., Montagne, A. & Zlokovic, B. V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434 (2017).
Hartmann, D. A. et al. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2, 041402 (2015).
Zhao, Z., Nelson, A. R., Betsholtz, C. & Zlokovic, B. V. Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078 (2015).
Sweeney, M. D., Ayyadurai, S. & Zlokovic, B. V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Montagne, A., Zhao, Z. & Zlokovic, B. V. Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J. Exp. Med. 214, 3151–3169 (2017).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018). This is the first study to examine molecular landmarks and heterogeneity of cerebrovascular cells in the adult mouse brain and investigate the concept of zonation along the arterio–capillary–venous axis.
Jack, C. R. Jr. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013). This study proposed and recognized hypothetical model of biomarker changes during Alzheimer’s disease pathophysiological progression.
Nguyen, L. N. et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506 (2014).
Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511 (2014).
Zeisel, A. et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Alarcon-Martinez, L. et al. Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. eLife 7, e34861 (2018).
Kisler, K. et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 20, 406–416 (2017). This was the first study to demonstrate that pericyte degeneration in a pericyte loss-of-function model leads to a loss of neurovascular coupling and diminished oxygen delivery to brain.
Peppiatt, C. M., Howarth, C., Mobbs, P. & Attwell, D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704 (2006). This important work in brain slices and retina demonstrated pericyte contractility and control of capillary diameter.
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014). This was the first study to show that pericytes have an active role in cerebral blood flow regulation in vivo and that capillaries can dilate ahead of arterioles. In ischemic conditions, pericytes rapidly constrict capillaries and die, consistent with the no-reflow phenomenon observed in stroke.
Mishra, A. et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 19, 1619–1627 (2016). This was the first study to show that astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles in brain, which involves a rise of calcium in astrocytes caused by entry through adenosine triphosphate-gated channels.
Biesecker, K. R. et al. Glial cell calcium signaling mediates capillary regulation of blood flow in the retina. J. Neurosci. 36, 9435–9445 (2016).
Fernández-Klett, F., Offenhauser, N., Dirnagl, U., Priller, J. & Lindauer, U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl. Acad. Sci. USA 107, 22290–22295 (2010).
Shih, A. & Hartmann, D. In vivo optical imaging and manipulation of pericytes in the mouse brain. in Optics and the Brain BrW3B–1 (2017).
Mapelli, L. et al. Granular layer neurons control cerebellar neurovascular coupling through an NMDA receptor/NO-dependent system. J. Neurosci. 37, 1340–1351 (2017).
Hill, R. A. et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110 (2015).
Wei, H. S. et al. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron 91, 851–862 (2016).
Hillman, E. M. C. Coupling mechanism and significance of the BOLD signal: a status report. Annu. Rev. Neurosci. 37, 161–181 (2014).
Longden, T. A. et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 20, 717–726 (2017).
Blinder, P. et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 16, 889–897 (2013).
Kur, J. & Newman, E. A. Purinergic control of vascular tone in the retina. J. Physiol. (Lond.) 592, 491–504 (2014).
Uhlirova, H. et al. Cell type specificity of neurovascular coupling in cerebral cortex. eLife 5, e14315 (2016).
Gebremedhin, D. et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ. Res. 87, 60–65 (2000).
Nippert, A. R., Biesecker, K. R. & Newman, E. A. Mechanisms mediating functional hyperemia in the brain. Neuroscientist 24, 73–83 (2018).
Bény, J.-L., Nguyen, M. N., Marino, M. & Matsui, M. Muscarinic receptor knockout mice confirm involvement of M3 receptor in endothelium-dependent vasodilatation in mouse arteries. J. Cardiovasc. Pharmacol. 51, 505–512 (2008).
Yamada, M. et al. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 98, 14096–14101 (2001).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Bell, R. D. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68, 409–427 (2010). This study not only describes the role of pericytes in maintaining in vivo blood–brain barrier integrity, microvascular density, and functional hyperemia during adulthood and brain aging but also shows that a primary loss of pericytes may lead to two parallel pathways of neurodegeneration, blood–brain barrier breakdown, and hypoperfusion, which lead to secondary neurodegenerative changes.
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Nikolakopoulou, A. M., Zhao, Z., Montagne, A. & Zlokovic, B. V. Regional early and progressive loss of brain pericytes but not vascular smooth muscle cells in adult mice with disrupted platelet-derived growth factor receptor-β signaling. PLoS One 12, e0176225 (2017).
Foo, S. S. et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124, 161–173 (2006).
Yao, Y., Chen, Z.-L., Norris, E. H. & Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 5, 3413 (2014).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516 (2012). This important study demonstrates APOE4-dependent activation of a pro-inflammatory signaling pathway in pericytes involving cyclophilin A–nuclear factor-kappa B–matrix metalloproteinase-9-mediated degradation of blood–brain barrier basement membrane and tight junction proteins.
Wang, Y., Chang, H., Rattner, A. & Nathans, J. Frizzled receptors in development and disease. Curr. Top. Dev. Biol. 117, 113–139 (2016).
Liebner, S. et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417 (2008).
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M. & Evans, A. C. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 (2016).
Montagne, A. et al. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 131, 687–707 (2016).
Montagne, A. et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015). This study was the first to demonstrate age-associated blood–brain barrier breakdown in the hippocampus in the living human brain and accelerated breakdown in humans with mild cognitive impairment.
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Arvanitakis, Z., Capuano, A. W., Leurgans, S. E., Bennett, D. A. & Schneider, J. A. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 15, 934–943 (2016). This large cross-sectional neuropathological study showed that cerebral vessel disease plays a part in dementia that is typically attributed to Alzheimer’s disease during life.
Toledo, J. B. et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136, 2697–2706 (2013).
Iadecola, C. The pathobiology of vascular dementia. Neuron 80, 844–866 (2013).
Rabin, J.S. et al. Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the Harvard Aging Brain Study. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2018.1123 (2018).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease. J. Cereb. Blood Flow Metab. 35, 1055–1068 (2015).
Ruitenberg, A. et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann. Neurol. 57, 789–794 (2005). This large population-based study showed that diminished cerebral blood flow precedes cognitive decline and hippocampal atrophy.
Chen, Y. et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 77, 1977–1985 (2011).
Hays, C. C., Zlatar, Z. Z. & Wierenga, C. E. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell. Mol. Neurobiol. 36, 167–179 (2016).
Wierenga, C. E. et al. Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. J. Cereb. Blood Flow Metab. 32, 1589–1599 (2012).
Alexopoulos, P. et al. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer’s disease measured by pulsed arterial spin labeling MRI. Eur. Arch. Psychiatry Clin. Neurosci. 262, 69–77 (2012).
Dai, W. et al. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250, 856–866 (2009).
Hu, W. T. et al. Distinct cerebral perfusion patterns in FTLD and AD. Neurology 75, 881–888 (2010).
Nation, D. A. et al. Cortical and subcortical cerebrovascular resistance index in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 36, 689–698 (2013).
van de Haar, H. J. et al. Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging 45, 190–196 (2016).
Yoshiura, T. et al. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. AJNR Am. J. Neuroradiol. 30, 1388–1393 (2009).
Leeuwis, A. E. et al. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimers Dement. 13, 531–540 (2017).
Ma, H. R. et al. Aberrant pattern of regional cerebral blood flow in Alzheimer’s disease: a voxel-wise meta-analysis of arterial spin labeling MR imaging studies. Oncotarget 8, 93196–93208 (2017).
de Eulate, R. G. et al. Reduced cerebral blood flow in mild cognitive impairment assessed using phase-contrast MRI. J. Alzheimers Dis. 58, 585–595 (2017).
Leijenaar, J. F. et al. Lower cerebral blood flow in subjects with Alzheimer’s dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimers Dement. 9, 76–83 (2017).
Wirth, M. et al. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. J. Cereb. Blood Flow Metab. https://doi.org/10.1177/2F0271678X16641128 (2016).
Michels, L. et al. Arterial spin labeling imaging reveals widespread and Aβ-independent reductions in cerebral blood flow in elderly apolipoprotein epsilon-4 carriers. J. Cereb. Blood Flow Metab. 36, 581–595 (2016).
Thambisetty, M., Beason-Held, L., An, Y., Kraut, M. A. & Resnick, S. M. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch. Neurol. 67, 93–98 (2010).
den Abeelen, A. S. S. M., Lagro, J., van Beek, A. H. E. A. & Claassen, J. A. H. R. Impaired cerebral autoregulation and vasomotor reactivity in sporadic Alzheimer’s disease. Curr. Alzheimer Res. 11, 11–17 (2014).
Yezhuvath, U. S. et al. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer’s disease. Neurobiol. Aging 33, 75–82 (2012).
Hecht, M., Krämer, L. M., von Arnim, C. A. F., Otto, M. & Thal, D. R. Capillary cerebral amyloid angiopathy in Alzheimer’s disease: association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol. 135, 681–694 (2018).
Suri, S. et al. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement. 11, 648–57.e1 (2015).
Hajjar, I., Sorond, F. & Lipsitz, L. A. Apolipoprotein E, carbon dioxide vasoreactivity, and cognition in older adults: effect of hypertension. J. Am. Geriatr. Soc. 63, 276–281 (2015).
Small, S. A., Perera, G. M., DeLaPaz, R., Mayeux, R. & Stern, Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann. Neurol. 45, 466–472 (1999).
Rombouts, S. A. et al. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am. J. Neuroradiol. 21, 1869–1875 (2000).
Dumas, A. et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann. Neurol. 72, 76–81 (2012).
Iadecola, C. et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat. Neurosci. 2, 157–161 (1999).
Niwa, K. et al. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc. Natl. Acad. Sci. USA 97, 9735–9740 (2000). This paper was the first to demonstrate altered neurovascular coupling by amyloid-β.
Park, L. et al. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 45, 1815–1821 (2014).
Montagne, A. et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat. Med. 24, 326–337 (2018).
Alata, W., Ye, Y., St-Amour, I., Vandal, M. & Calon, F. Human apolipoprotein E ɛ4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J. Cereb. Blood Flow Metab. 35, 86–94 (2015).
van de Haar, H. J. et al. Blood-brain barrier leakage in patients with Early Alzheimer disease. Radiology 281, 527–535 (2016).
van de Haar, H. J. et al. Subtle blood-brain barrier leakage rate and spatial extent: Considerations for dynamic contrast-enhanced MRI. Med. Phys. 44, 4112–4125 (2017).
Heringa, S. M. et al. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J. Alzheimers Dis. 38, 211–221 (2014).
Poliakova, T., Levin, O., Arablinskiy, A., Vasenina, E. & Zerr, I. Cerebral microbleeds in early Alzheimer’s disease. J. Neurol. 263, 1961–1968 (2016).
Shams, S. et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the Karolinska Imaging Dementia Study. AJNR Am. J. Neuroradiol. 36, 661–666 (2015).
Shams, S. & Wahlund, L.-O. Cerebral microbleeds as a biomarker in Alzheimer’s disease? A review in the field. Biomark. Med. 10, 9–18 (2016).
Vernooij, M. W. et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 70, 1208–1214 (2008).
Yates, P. A. et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 82, 1266–1273 (2014).
Brundel, M. et al. High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s disease. J. Alzheimers Dis. 31, 259–263 (2012). A high prevalence of cerebral microbleeds is detected in mild cognitive impairment and early Alzheimer’s disease brains (78% of subjects) using state-of-the-art 7-Tesla magnetic resonance imaging.
Uetani, H. et al. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am. J. Neuroradiol. 34, 984–989 (2013).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838 (2013).
Saito, S. & Ihara, M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr. Opin. Psychiatry 29, 168–173 (2016).
Iaccarino, L. et al. A cross-validation of FDG- and amyloid-PET biomarkers in mild cognitive impairment for the risk prediction to dementia due to Alzheimer’s disease in a clinical setting. J. Alzheimers Dis. 59, 603–614 (2017).
Mosconi, L. et al. Amyloid and metabolic positron emission tomography imaging of cognitively normal adults with Alzheimer’s parents. Neurobiol. Aging 34, 22–34 (2013).
Mosconi, L. et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J. Nucl. Med. 49, 390–398 (2008).
Protas, H. D. et al. Posterior cingulate glucose metabolism, hippocampal glucose metabolism, and hippocampal volume in cognitively normal, late-middle-aged persons at 3 levels of genetic risk for Alzheimer disease. JAMA Neurol. 70, 320–325 (2013).
Mosconi, L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur. J. Nucl. Med. Mol. Imaging 32, 486–510 (2005).
Bailly, M. et al. Precuneus and cingulate cortex atrophy and hypometabolism in patients with Alzheimer’s disease and mild cognitive impairment: MRI and (18)F-FDG PET quantitative analysis using FreeSurfer. BioMed Res. Int. 2015, 583931 (2015).
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804 (2012).
Mosconi, L. et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J. Nucl. Med. 47, 1778–1786 (2006).
Landau, S. M. et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging 32, 1207–1218 (2011).
Landau, S. M. et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75, 230–238 (2010).
Mosconi, L. et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 36, 811–822 (2009).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Winkler, E. A. et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 18, 521–530 (2015).
Gejl, M. et al. Blood-brain glucose transfer in Alzheimer’s disease: effect of GLP-1 analog treatment. Sci. Rep. 7, 17490 (2017).
Jagust, W. J. et al. Diminished glucose transport in Alzheimer’s disease: dynamic PET studies. J. Cereb. Blood Flow Metab. 11, 323–330 (1991).
Piert, M., Koeppe, R. A., Giordani, B., Berent, S. & Kuhl, D. E. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J. Nucl. Med. 37, 201–208 (1996).
Mooradian, A. D., Chung, H. C. & Shah, G. N. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol. Aging 18, 469–474 (1997).
Kalaria, R. N. & Harik, S. I. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J. Neurochem. 53, 1083–1088 (1989).
Simpson, I. A., Chundu, K. R., Davies-Hill, T., Honer, W. G. & Davies, P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer’s disease. Ann. Neurol. 35, 546–551 (1994).
Horwood, N. & Davies, D. C. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer’s disease. Virchows Arch. 425, 69–72 (1994).
Kilbourn, M. R. Small molecule PET tracers for transporter imaging. Semin. Nucl. Med. 47, 536–552 (2017).
Cirrito, J. R. et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest. 115, 3285–3290 (2005).
Deo, A. K. et al. Activity of P-glycoprotein, a β-amyloid transporter at the blood-brain barrier, is compromised in patients with mild Alzheimer disease. J. Nucl. Med. 55, 1106–1111 (2014).
van Assema, D. M. E. et al. Blood-brain barrier P-glycoprotein function in Alzheimer’s disease. Brain 135, 181–189 (2012).
Sagare, A. P. et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 4, 2932 (2013).
Paul, J., Strickland, S. & Melchor, J. P. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J. Exp. Med. 204, 1999–2008 (2007).
Deane, R. et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 9, 907–913 (2003).
Deane, R. et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43, 333–344 (2004).
Deane, R. et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 122, 1377–1392 (2012).
Gama Sosa, M. A. et al. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am. J. Pathol. 176, 353–368 (2010).
Wen, P. H. et al. Selective expression of presenilin 1 in neural progenitor cells rescues the cerebral hemorrhages and cortical lamination defects in presenilin 1-null mutant mice. Development 132, 3873–3883 (2005).
Blair, L. J. et al. Tau depletion prevents progressive blood-brain barrier damage in a mouse model of tauopathy. Acta Neuropathol. Commun. 3, 8 (2015).
Cacciottolo, M. et al. The APOE4 allele shows opposite sex bias in microbleeds and Alzheimer’s disease of humans and mice. Neurobiol. Aging 37, 47–57 (2016).
Melzer, T. R. et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain 134, 845–855 (2011).
Syrimi, Z. J. et al. Arterial spin labelling detects posterior cortical hypoperfusion in non-demented patients with Parkinson’s disease. J. Neural Transm. (Vienna) 124, 551–557 (2017).
Chen, J. J., Salat, D. H. & Rosas, H. D. Complex relationships between cerebral blood flow and brain atrophy in early Huntington’s disease. Neuroimage 59, 1043–1051 (2012).
Ingrisch, M. et al. Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Invest. Radiol. 47, 252–258 (2012).
Hojjat, S.-P. et al. Cortical perfusion alteration in normal-appearing gray matter is most sensitive to disease progression in relapsing-remitting multiple sclerosis. AJNR Am. J. Neuroradiol. 37, 1454–1461 (2016).
Murphy, M. J. et al. Widespread cerebral haemodynamics disturbances occur early in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 13, 202–209 (2012).
Rule, R. R., Schuff, N., Miller, R. G. & Weiner, M. W. Gray matter perfusion correlates with disease severity in ALS. Neurology 74, 821–827 (2010).
Camargo, C. H. F. et al. Abnormal cerebrovascular reactivity in patients with Parkinson’s disease. Parkinsons Dis. 2015, 523041 (2015).
Al-Bachari, S., Vidyasagar, R., Emsley, H. C. & Parkes, L. M. Structural and physiological neurovascular changes in idiopathic Parkinson’s disease and its clinical phenotypes. J. Cereb. Blood Flow Metab. 37, 3409–3421 (2017).
Marshall, O., Chawla, S., Lu, H., Pape, L. & Ge, Y. Cerebral blood flow modulation insufficiency in brain networks in multiple sclerosis: A hypercapnia MRI study. J. Cereb. Blood Flow Metab. 36, 2087–2095 (2016).
Al-Bachari, S. MRI assessment of neurovascular changes in idiopathic Parkinson’s disease. Doctoral thesis, University of Manchester (2016).
Drouin-Ouellet, J. et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann. Neurol. 78, 160–177 (2015). Blood–brain barrier leakage and cerebrovascular dysfunction in Huntington’s disease investigated by neuroimaging and postmortem tissue analysis in human subjects and further corroborated in a mouse model of Huntington’s disease.
Taheri, S., Gasparovic, C., Shah, N. J. & Rosenberg, G. A. Quantitative measurement of blood-brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn. Reson. Med. 65, 1036–1042 (2011).
Cramer, S. P., Simonsen, H., Frederiksen, J. L., Rostrup, E. & Larsson, H. B. W. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin. 4, 182–189 (2013).
Gaitán, M. I. et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Ann. Neurol. 70, 22–29 (2011).
Ham, J. H. et al. Cerebral microbleeds in patients with Parkinson’s disease. J. Neurol. 261, 1628–1635 (2014).
Kim, J.-H., Park, J., Kim, Y.-H., Ma, H.-I. & Kim, Y. J. Characterization of cerebral microbleeds in idiopathic Parkinson’s disease. Eur. J. Neurol. 22, 377–383 (2015).
Yamashiro, K. et al. The prevalence and risk factors of cerebral microbleeds in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 21, 1076–1081 (2015).
Kwan, J. Y. et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7, e35241 (2012).
Kortekaas, R. et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann. Neurol. 57, 176–179 (2005).
Gerwien, H. et al. Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood-brain barrier. Sci. Transl. Med. 8, 364ra152 (2016). The authors developed an important, novel approach to image initial leukocyte penetration of the blood–brain barrier in multiple sclerosis patients and animal models.
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011).
Nelson, A. R., Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta 1862, 887–900 (2016).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 5, 347–360 (2004).
Montagne, A. et al. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. Neuroimage 63, 760–770 (2012).
Acknowledgements
The work of B.V.Z. is supported by the National Institutes of Health grants R01AG023084, R01NS090904, R01NS034467, R01AG039452, 1R01NS100459, 5P01AG052350, and 5P50AG005142, in addition to the Alzheimer’s Association, Cure Alzheimer’s Fund, and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference no. 16 CVD 05. We apologize to those authors whose excellent original papers we were not able to cite due to space limitations; instead, we sometimes cited recent reviews by leading authorities so that the reader can access all key primary papers in the field of our review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sweeney, M.D., Kisler, K., Montagne, A. et al. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 21, 1318–1331 (2018). https://doi.org/10.1038/s41593-018-0234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0234-x
This article is cited by
-
Vascular dysfunction in sporadic bvFTD: white matter hyperintensity and peripheral vascular biomarkers
Alzheimer's Research & Therapy (2024)
-
Platelet-derived growth factor signaling in pericytes promotes hypothalamic inflammation and obesity
Molecular Medicine (2024)
-
Sex, hormones and cerebrovascular function: from development to disorder
Fluids and Barriers of the CNS (2024)
-
Blockage of VEGF function by bevacizumab alleviates early-stage cerebrovascular dysfunction and improves cognitive function in a mouse model of Alzheimer’s disease
Translational Neurodegeneration (2024)
-
Effects of fetal growth restriction on the perinatal neurovascular unit and possible treatment targets
Pediatric Research (2024)