Abstract
To uncover the circuit-level alterations that underlie atypical sensory processing associated with autism, we adopted a symptom-to-circuit approach in the Fmr1-knockout (Fmr1–/–) mouse model of Fragile X syndrome. Using a go/no-go task and in vivo two-photon calcium imaging, we find that impaired visual discrimination in Fmr1–/– mice correlates with marked deficits in orientation tuning of principal neurons and with a decrease in the activity of parvalbumin interneurons in primary visual cortex. Restoring visually evoked activity in parvalbumin cells in Fmr1–/– mice with a chemogenetic strategy using designer receptors exclusively activated by designer drugs was sufficient to rescue their behavioral performance. Strikingly, human subjects with Fragile X syndrome exhibit impairments in visual discrimination similar to those in Fmr1–/– mice. These results suggest that manipulating inhibition may help sensory processing in Fragile X syndrome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the analyzed data reported in this study is available from the corresponding author upon reasonable request.
Change history
15 November 2018
The original and corrected Acknowledgements are shown in the accompanying Author Correction.
26 July 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41593-023-01402-8
References
Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), Edn. 5th. (2013).
Robertson, C. E. & Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 18, 671–684 (2017).
Estes, A. et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 7, 24 (2015).
Rifé, M. et al. Incidence of Fragile X in 5,000 consecutive newborn males. Genet. Test. 7, 339–343 (2003).
The Dutch-Belgian Fragile X Consortium. Fmr1 knockout mice: a model to study Fragile X mental retardation. Cell 78, 23–33 (1994).
Contractor, A., Klyachko, V. A. & Portera-Cailliau, C. Altered neuronal and circuit excitability in Fragile X syndrome. Neuron 87, 699–715 (2015).
He, C. & Portera-Cailliau, C. The trouble with spines in Fragile X syndrome: density, maturity and plasticity. Neuroscience 251, 120–128 (2013).
He, C. X. et al. Tactile defensiveness and impaired adaptation of neuronal activity in the Fmr1 knock-out mouse model of autism. J. Neurosci. 37, 6475–6487 (2017).
Farzin, F., Whitney, D., Hagerman, R. J. & Rivera, S. M. Contrast detection in infants with Fragile X syndrome. Vision Res. 48, 1471–1478 (2008).
Kogan, C. S. et al. Differential impact of the FMR1 gene on visual processing in Fragile X syndrome. Brain 127, 591–601 (2004).
Poort, J. et al. Learning enhances sensory and multiple non-sensory representations in primary visual cortex. Neuron 86, 1478–1490 (2015).
Lee, S. H. et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature 488, 379–383 (2012).
Zupan, B. & Toth, M. Wild-type male offspring of fmr-1+/- mothers exhibit characteristics of the Fragile X phenotype. Neuropsychopharmacology 33, 2667–2675 (2008).
Zhang, Y. et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat. Neurosci. 17, 1701–1709 (2014).
Arnett, M. T., Herman, D. H. & McGee, A. W. Deficits in tactile learning in a mouse model of Fragile X syndrome. PLoS One 9, e109116 (2014).
Juczewski, K. et al. Somatosensory map expansion and altered processing of tactile inputs in a mouse model of Fragile X syndrome. Neurobiol. Dis. 96, 201–215 (2016).
Gonçalves, J. T., Anstey, J. E., Golshani, P. & Portera-Cailliau, C. Circuit level defects in the developing neocortex of Fragile X mice. Nat. Neurosci. 16, 903–909 (2013).
La Fata, G. et al. FMRP regulates multipolar to bipolar transition affecting neuronal migration and cortical circuitry. Nat. Neurosci. 17, 1693–1700 (2014).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Gonchar, Y. & Burkhalter, A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Cortex 7, 347–358 (1997).
Zariwala, H. A. et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141 (2012).
Hofer, S. B. et al. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat. Neurosci. 14, 1045–1052 (2011).
Runyan, C. A. & Sur, M. Response selectivity is correlated to dendritic structure in parvalbumin-expressing inhibitory neurons in visual cortex. J. Neurosci. 33, 11724–11733 (2013).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007).
Mullard, A. Fragile X disappointments upset autism ambitions. Nat. Rev. Drug. Discov. 14, 151–153 (2015).
Atallah, B. V., Bruns, W., Carandini, M. & Scanziani, M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170 (2012).
Kato, H. K., Asinof, S. K. & Isaacson, J. S. Network-level control of frequency tuning in auditory cortex. Neuron 95, 412–423.e4 (2017).
Knoth, I. S., Vannasing, P., Major, P., Michaud, J. L. & Lippé, S. Alterations of visual and auditory evoked potentials in Fragile X syndrome. Int. J. Dev. Neurosci. 36, 90–97 (2014).
LeBlanc, J. J. et al. Visual evoked potentials detect cortical processing deficits in Rett syndrome. Ann. Neurol. 78, 775–786 (2015).
Niell, C. M. & Stryker, M. P. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010).
Pakan, J. M. et al. Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. eLife 5, e14985 (2016).
Dipoppa, M. et al. Vision and locomotion shape the interactions between neuron types in mouse visual cortex. Neuron 98, 602–615.e8 (2018).
Guo, Z. V. et al. Flow of cortical activity underlying a tactile decision in mice. Neuron 81, 179–194 (2014).
Mostany, R. & Portera-Cailliau, C. A craniotomy surgery procedure for chronic brain imaging. J. Vis. Exp. https://doi.org/10.3791/680 (2008).
Golshani, P. & Portera-Cailliau, C. In vivo 2-photon calcium imaging in layer 2/3 of mice. J. Vis. Exp. https://doi.org/10.3791/681 (2008).
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Mineault, P. J., Tring, E., Trachtenberg, J. T. & Ringach, D. L. Enhanced spatial resolution during locomotion and heightened attention in mouse primary visual cortex. J. Neurosci. 36, 6382–6392 (2016).
Cottam, J. C., Smith, S. L. & Häusser, M. Target-specific effects of somatostatin-expressing interneurons on neocortical visual processing. J. Neurosci. 33, 19567–19578 (2013).
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012).
Hooper, S. R. et al. Executive functions in young males with Fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology 22, 36–47 (2008).
Hamm, J. P., Peterka, D. S., Gogos, J. A. & Yuste, R. Altered cortical ensembles in mouse models of schizophrenia. Neuron 94, 153–167.e8 (2017).
Pinto, L. & Dan, Y. Cell-type-specific activity in prefrontal cortex during goal-directed behavior. Neuron 87, 437–450 (2015).
Harvey, C. D., Coen, P. & Tank, D. W. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 484, 62–68 (2012).
Kogan, C. S. et al. Integrative cortical dysfunction and pervasive motion perception deficit in Fragile X syndrome. Neurology 63, 1634–1639 (2004).
Plaisted, K., O’Riordan, M. & Baron-Cohen, S. Enhanced visual search for a conjunctive target in autism: a research note. J. Child Psychol. Psychiatry 39, 777–783 (1998).
Acknowledgements
The authors thank K. Cohan, S. Cohen, and M. Hong for help with early behavioral experiments; P. Golshani and M. Einstein for advice on mouse behavior; the Janelia GENIE project (for providing GCaMP6s); P. Yu for building custom lick ports; and D. Buonomano, A. Contractor, and J. Sweeney for feedback on the manuscript. The authors also thank P. Pellionisz and A. Cheng for help assembling the two-photon microscope. K. Battista created the illustration in Fig. 1b. This work was supported by the following grants: W81XWH-17-1-0231 (USAMRMC, DOD), Developmental Disabilities Translational Research Program #20160969 (John Merck), SFARI Award 295438 (Simons Foundation), and 5R01HD054453 (NICHD/NIH) awarded to C.P.-C.; K23 MH112936 (NIMH/NIH) to E.P.; a grant from the Fragile X Alliance of Ohio to C.A.E.; and U01 DD001185 (NCBDDD/NIH), U54 HD082008 (NICHD/NIH), and a grant from the Cincinnati Children’s Hospital Research Foundation to E.P. and C.A.E.
Author information
Authors and Affiliations
Contributions
A.G. and C.P-C. conceived the project and designed the experiments with help from J.G., L.M.S., E.P., and C.A.E. for the human studies. A.G. developed the behavioral experiment for mice and humans. A.G. and D.A.C. wrote the Matlab code for analysis. A.G, G.R.C., A.N., B.T., D.d.A., N.K., and J.G. conducted the experiments and analyzed the data. A.G., L.M.S., E.P., C.A.E., and C.P-C. interpreted the data and wrote the paper with input from other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Behavioral performance on visual discrimination task according to genotype (corresponds to data in Fig. 1d,e).
a, b. Discriminability index (d’) for WT (a, black) and Fmr1-/- mice (b, red). Each line represents a different mouse. The horizontal dashed line indicates a d’ = 2, which corresponds to 90% correct rejections.
Supplementary Figure 2 Fmr1–/– mice are delayed in suppressing the no-go response (corresponds to data in Fig. 1e).
a. Sample behavioral data for a representative WT mouse (top) and Fmr1-/- mouse (bottom) over 100 trials in session #1 vs. Session #4. Only ‘Hit’, ‘false alarm’ (FA) and ‘correct rejection (CR) responses are shown. Note how the WT animal is able to suppress the FA responses and increase the proportion of CR responses by session #4, whereas there is virtually no change in response characteristics for the Fmr1-/- animal between session #1 and session #4. However, by the learned session (session #7), the Fmr1-/- mouse has suppressed its FA responses. b. Profile of proportion of Hit, CR, FA and Miss responses over the course of training. Hit: F4,156 = 1.05, p = 0.03, CR: F4,156 = 2.29, p = 0.1 × 10-3; Miss: F4,156 = 2.99, p = 0.5 × 10-5; FA: F4,156 = 2.53, p = 0.4 × 10-5; Friedman test with repeated measures on training. Two-sided Mann-Whitney tests (genotypes), p-values shown on the Figure.
Supplementary Figure 3 Fmr1–/– mice do not slow down as much as WT mice during preferred stimuli (Hit responses) (corresponds to data in Fig. 1e,f).
a. Workflow for ball motion analysis. b. Analysis of ball motion was done with semi-automated, custom MATLAB scripts to detect black dots painted evenly onto the polystyrene ball. c. Discriminability index for a subset of the mice shown in Fig. 1d in which we recorded running speed. d-f. Overall running speed (as determined by ball motion analysis) in WT and Fmr1-/- mice at session #1 (d), session #4 (e) and final learned session (f) for Hit + Miss trials only (corresponding to preferred stimuli). We observed a significant difference in the degree to which WT and Fmr1-/- mice slow down at session #4, but not in the final learned session. Repeated measures ANOVA F2,16 = 5.49, p = 2 × 10-5. Note there is no significant genotype differences in running speed in session 1 or in the final learned session. g-i. Change in running speed (as above). F2,16 = 2.13, p = 0.02. Shaded area in panels d-i indicate s.e.m.
Supplementary Figure 4 No sex differences in task performance (corresponds to data in Fig. 1d).
There were no significant differences in task performance between n = mice, male (n = 22) and female mice (n = 18) of either genotype. χ23,37 = 11.7, p = 0.001, Kruskal-Wallis test (all comparisons); effect of sex: p = 0.20, two-sided Mann-Whitney; effect of genotype: p = 6.6 × 10-6, two-sided Mann-Whitney. Horizontal bars indicate mean and error bars indicate s.e.m.
Supplementary Figure 5 No deficits in task performance above > 10% contrast (corresponds to data in Fig. 1).
a. To investigate whether Fmr1-/- mice have impairments in basic visual discrimination, we randomly presented drifting gratings of varying contrast, from 0% to 100%, and tested mice of both genotypes that had already reached a d’ > 2 at 100% contrast on the visual discrimination task with 90o as the angle difference between preferred and non-preferred directions. b. Representative cumulative histograms of % correct responses from a WT and a Fmr1-/- mouse at different contrasts (data in panels b-d is for mice after they learned the basic 90o task). c. There were no significant differences in task performance between WT and Fmr1-/- mice when the contrast for gratings was > 10%. p = 0.6, Two-sided Mann-Whitney test.n = mice, Fmr1-/-(5), WT (4). d. Mice that had learned the task at 100% contrast then performed poorly (d’ < 2) below a certain contrast threshold and Fmr1-/- mice had a higher contrast threshold than WT mice. n = mice, Fmr1-/-(5), WT (4). p = 0.043, Two-sided Mann-Whitney test.
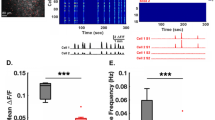
Supplementary Figure 6 Calcium imaging of spontaneous activity in pyramidal neurons† in V1 (corresponds to data in Fig. 3).
a. There was no significant difference in the amplitude of the calcium signals (as assessed with the mean Z-score of fluorescence) of pyramidal neurons in L2/3 of V1 cortex between WT and Fmr1-/- mice during recordings of spontaneous activity. Unpaired two-tailed Student’s t-test for panels a-b. In both panels horizontal bars indicate the mean and error bars indicate s.e.m. b. There was no significant difference in the frequency of peaks of calcium fluorescence of pyramidal neurons in L2/3 of V1 cortex between WT and Fmr1-/- mice during recordings of spontaneous activity. †In this legend, the term ‘pyramidal’ refers to all cells recorded using GCaMP6s, the vast majority of which are pyramidal neurons.
Supplementary Figure 7 The tuning width of pyramidal neurons correlates with task performance (corresponds to data in Fig. 3h).
The performance of mice on the visual discrimination task correlates with the tuning width of pyramidal neurons, r = 0.48, p = 0.041. Pearson’s correlation.
Supplementary Figure 8 Calcium imaging of spontaneous activity in PV neurons in V1 (corresponds to data in Fig. 4).
a. There was no significant difference in the overall frequency of calcium transients (as measured by the mean fluorescence Z-scores per s) of PV neurons in L2/3 between WT and Fmr1-/- mice during recordings of spontaneous activity in V1 (unpaired two-tailed Student’s t-test). n = mice. Fmr1-/-(5), WT (6). b. There was a non-significant trend for lower frequency of peaks of calcium fluorescence of PV neurons in L2/3 of Fmr1-/- mice compared to WT mice, during recordings of spontaneous activity in V1 (two-sided Mann-Whitney test). n = mice. Fmr1-/-(5), WT (6). c. Fmr1-/- mice showed a significantly lower correlation between the activity of PV cells (as assessed by their fluorescence calcium signals) and the epochs of visual stimulation, compared to WT mice, p = 0.0005 (two-sided Mann-Whitney test). n = mice. Fmr1-/-(6), WT (7). In all 3 panels horizontal bars indicate the mean and error bars indicate s.e.m.
Supplementary Figure 9 Controls for DREADD experiments (corresponds to data in Fig. 5).
a-d. DREADD expression does not alter activity of PV cells, as we observed no differences in visual evoked calcium transients (as measured by the mean fluorescence Z-scores per s) (a) or frequency of visually evoked peaks of activity (b) or in the number of active PV cells (c), or the fraction of PV cells that responded to visual stimuli (d) between Fmr1-/- mice and Fmr1-/-, hM3Dq mice (before CNO administration). Two-sided Mann-Whitney test for panels a-b and unpaired two-tailed student’s t-test for panels c-d. In all the panels horizontal bars indicate the mean and error bars indicate s.e.m.
Supplementary Figure 10 No significant effect of hM3Dq and CNO on spontaneous PV cell activity (corresponds to data in Fig. 5a–e).
a,b. In PV-Cre x Fmr1-/- mice injected with the hM3Dq DREADD virus in V1, CNO injection led to no significant changes in either the overall frequency of calcium transients (as measured by the mean fluorescence Z-scores per s) or the frequency of calcium peaks for PV cells. n = mice, Fmr1-/-, HM3Dq (before and after CNO) (6). Unpaired two-tailed student’s t-test. In both panels horizontal bars indicate the mean and error bars indicate s.e.m.
Supplementary Figure 11 Correlation between task performance and PV cells (corresponds to data in Fig. 5).
There is a strong inverse correlation between task performance (days to reach d’ > 2) and the fraction of stimulus-responsive PV cells in V1, r -0.753, p = 0.0007. Pearson’s correlation.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11
Rights and permissions
About this article
Cite this article
Goel, A., Cantu, D.A., Guilfoyle, J. et al. Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat Neurosci 21, 1404–1411 (2018). https://doi.org/10.1038/s41593-018-0231-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0231-0
This article is cited by
-
Deletion of Fmr1 in parvalbumin-expressing neurons results in dysregulated translation and selective behavioral deficits associated with fragile X syndrome
Molecular Autism (2022)
-
Haploinsufficiency of Shank3 increases the orientation selectivity of V1 neurons
Scientific Reports (2022)
-
Neocortical localization and thalamocortical modulation of neuronal hyperexcitability contribute to Fragile X Syndrome
Communications Biology (2022)
-
Two-photon calcium imaging of neuronal activity
Nature Reviews Methods Primers (2022)
-
Restoration of FMRP expression in adult V1 neurons rescues visual deficits in a mouse model of fragile X syndrome
Protein & Cell (2022)