Abstract
The paraventricular nucleus of the thalamus (PVT) is increasingly being recognized as a critical node linking stress detection to the emergence of adaptive behavioral responses to stress. However, despite growing evidence implicating the PVT in stress processing, the neural mechanisms by which stress impacts PVT neurocircuitry and promotes stressed states remain unknown. Here we show that stress exposure drives a rapid and persistent reduction of inhibitory transmission onto projection neurons of the posterior PVT (pPVT). This stress-induced disinhibition of the pPVT was associated with a locus coeruleus-mediated rise in the extracellular concentration of dopamine in the midline thalamus, required the function of dopamine D2 receptors on PVT neurons, and increased sensitivity to stress. Our findings define the locus coeruleus as an important modulator of PVT function: by controlling the inhibitory tone of the pPVT, it modulates the excitability of pPVT projection neurons and controls stress responsivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vertes, R. P., Linley, S. B. & Hoover, W. B. Limbic circuitry of the midline thalamus. Neurosci. Biobehav. Rev. 54, 89–107 (2015).
Hsu, D. T., Kirouac, G. J., Zubieta, J. K. & Bhatnagar, S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front. Behav. Neurosci. 8, 73 (2014).
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015).
Do-Monte, F. H., Quiñones-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015).
Zhu, Y., Wienecke, C. F., Nachtrab, G. & Chen, X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222 (2016).
Do-Monte, F. H., Minier-Toribio, A., Quiñones-Laracuente, K., Medina-Colón, E. M. & Quirk, G. J. Thalamic regulation of sucrose seeking during unexpected reward omission. Neuron 94, 388–400.e4 (2017).
Arcelli, P., Frassoni, C., Regondi, M. C., De Biasi, S. & Spreafico, R. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res. Bull. 42, 27–37 (1997).
Zhang, X. & van den Pol, A. N. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356, 853–859 (2017).
Betley, J. N., Cao, Z. F., Ritola, K. D. & Sternson, S. M. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350 (2013).
Giber, K. et al. A subcortical inhibitory signal for behavioral arrest in the thalamus. Nat. Neurosci. 18, 562–568 (2015).
Bodor, A. L., Giber, K., Rovó, Z., Ulbert, I. & Acsády, L. Structural correlates of efficient GABAergic transmission in the basal ganglia-thalamus pathway. J. Neurosci. 28, 3090–3102 (2008).
Wanaverbecq, N. et al. Contrasting the functional properties of GABAergic axon terminals with single and multiple synapses in the thalamus. J. Neurosci. 28, 11848–11861 (2008).
Halassa, M. M. & Acsády, L. Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci. 39, 680–693 (2016).
Del Castillo, J. & Katz, B. Quantal components of the end-plate potential. J. Physiol. (Lond.) 124, 560–573 (1954).
Redman, S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol. Rev. 70, 165–198 (1990).
Stevens, C. F. Quantal release of neurotransmitter and long-term potentiation. Cell 72 Suppl, 55–63 (1993).
Goldstein, J. M., Jerram, M., Abbs, B., Whitfield-Gabrieli, S. & Makris, N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 30, 431–438 (2010).
Ramikie, T. S. & Ressler, K. J. Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol. Psychiatry 83, 876–885 (2018).
Grimley, J. S. et al. Visualization of synaptic inhibition with an optogenetic sensor developed by cell-free protein engineering automation. J. Neurosci. 33, 16297–16309 (2013).
Dong, X., Li, S. & Kirouac, G. J. Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct. Funct. 222, 3927–3943 (2017).
Wimmer, R. D. et al. Thalamic control of sensory selection in divided attention. Nature 526, 705–709 (2015).
Dobbs, L. K. et al. Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron 90, 1100–1113 (2016).
Floran, B., Floran, L., Sierra, A. & Aceves, J. D2 receptor-mediated inhibition of GABA release by endogenous dopamine in the rat globus pallidus. Neurosci. Lett. 237, 1–4 (1997).
Lalchandani, R. R., van der Goes, M. S., Partridge, J. G. & Vicini, S. Dopamine D2 receptors regulate collateral inhibition between striatal medium spiny neurons. J. Neurosci. 33, 14075–14086 (2013).
Li, S., Shi, Y. & Kirouac, G. J. The hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Front. Neuroanat. 8, 136 (2014).
Kempadoo, K. A., Mosharov, E. V., Choi, S. J., Sulzer, D. & Kandel, E. R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 113, 14835–14840 (2016).
Takeuchi, T. et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016).
Gerfen, C. R., Paletzki, R. & Heintz, N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013).
Abercrombie, E. D., Keller, R. W. Jr. & Zigmond, M. J. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience 27, 897–904 (1988).
Kirouac, G. J. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev. 56, 315–329 (2015).
Moruzzi, G. & Magoun, H. W. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1, 455–473 (1949).
Cullinan, W. E., Herman, J. P., Battaglia, D. F., Akil, H. & Watson, S. J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505 (1995).
Dallman, M. F. et al. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann. NY Acad. Sci. 1018, 141–150 (2004).
Oh, W. C., Lutzu, S., Castillo, P. E. & Kwon, H. B. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science 353, 1037–1040 10 (2016).
Chen, J. L. et al. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74, 361–373 (2012).
Villa, K. L. et al. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 90, 662–664 (2016).
Li, Y. et al. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl.) 212, 251–265 (2010).
Takada, M., Campbell, K. J., Moriizumi, T. & Hattori, T. On the origin of the dopaminergic innervation of the paraventricular thalamic nucleus. Neurosci. Lett. 115, 33–36 (1990).
Uematsu, A. et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 20, 1602–1611 (2017).
Berridge, C. W. & Waterhouse, B. D. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 42, 33–84 (2003).
Martins, A. R. & Froemke, R. C. Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat. Neurosci. 18, 1483–1492 (2015).
Schmitt, L. I. et al. Thalamic amplification of cortical connectivity sustains attentional control. Nature 545, 219–223 (2017).
Sherman, S. M. Thalamus plays a central role in ongoing cortical functioning. Nat. Neurosci. 19, 533–541 (2016).
Sherman, S.M. & Guillery, R.W. Exploring the Thalamus. (Academic Press, Cambridge, MA, 2001).
McCall, J. G. et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Bingham, B. et al. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology 36, 896–909 (2011).
Curtis, A. L., Lechner, S. M., Pavcovich, L. A. & Valentino, R. J. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J. Pharmacol. Exp. Ther. 281, 163–172 (1997).
Skirzewski, M. et al. ErbB4 signaling in dopaminergic axonal projections increases extracellular dopamine levels and regulates spatial/working memory behaviors. Mol. Psychiatry https://doi.org/10.1038/mp.2017.132 (2017).
Acknowledgements
We thank T. Davidson (Stanford University) for his assistance in the design of the fiber photometry system and G. Augustine (Lee Kong Chian School of Medicine, Singapore) for the SuperClomeleon plasmid. This work was supported by the NIMH Intramural Research Program (M.A.P.), NICHD Intramural Research Program (A.B.), and NIH Grant MH107460 (H.-B.K.).
Author information
Authors and Affiliations
Contributions
B.S.B. performed anatomical and immunohistochemical studies, calcium and chloride imaging experiments, optogenetic experiments, and stereotaxic injections for all experiments. B.J.W. and M.A.P. performed electrophysiological experiments. M.S. performed microdialysis experiments and analyzed collected fractions via HPLC. Y.L. performed RT-PCR and in situ hybridization experiments. J.H.H. performed all procedures for two-photon imaging of gephyrin puncta. O.K. and N.R. performed stereotaxic surgeries and histological procedures. A.B. and H.-B.K. provided critical reagents and suggestions. B.S.B., B.J.W., and M.A.P. designed the study, interpreted results, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Inhibitory projections to the pPVT.
Compilation of data from the Mouse Brain Connectivity Atlas of the Allen Brain Institute (connectivity.brain-map.org) showing eight different areas of the mouse brain where a Cre-dependent AAV expressing GFP was injected (left panels), and fluorescently-labelled terminals were detected in the pPVT (right panels). Abbreviations: MPO, medial preoptic area; SI, substantia innominata; AHN, anterior hypothalamic nucleus; DMH, dorsomedial hypothalamus; ZI, zona incerta; PAG, periaqueductal gray; MRN, median raphe nucleus; PRNo, pontine reticular nucleus oral part; PRNc, pontine reticular nucleus caudal part. The following mouse Cre-lines were used for these experiments: GAD2-IRES-Cre (MPO, SI, AHN, DMH, ZI, PAG), Slc32a1-IRES-Cre (MRN/PRNo), and Slc6a5-Cre (PRNc).
Supplementary Figure 2 Most neurons of the pPVT express dopamine D2 receptors.
Representative images showing coincident expression of D2-EGFP and the neuronal marker NeuN in the pPVT of wildtype mice (colocalization: 70% D2+/NeuN, n = 3 mice).
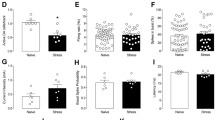
Supplementary Figure 3 Sex differences do not account for stress-induced disinhibition of projection neurons of pPVT.
Quantification of mIPSC amplitude and frequency analyzed across both sexes (blue- males; pink- females). Amplitude in pA, naïve male, 49.93 ± 3.62, n = 17 neurons, 2 mice; naïve female, 49.08 ± 4.83, n = 14 neurons, 2 mice; two-sided t-test, non-significant, P = 0.89; restraint male, 39.05 ± 4.66, n = 7 neurons, 2 mice; restraint female, 36.64 ± 1.07, n = 17 neurons, 2 mice; two-sided t-test, non-significant, P = 0.48; footshock male, 41.93 ± 2.26, n = 21 neurons, 2 mice; footshock female, 40.76 ± 1.83, n = 30 neurons, 2 mice; two-sided t-test, non-significant, P = 0.69. Frequency in Hz, naïve male, 5.51 ± 1.06, n = 17 neurons; naïve female, 4.34 ± 0.73, n = 14 neurons, 2 mice; two-sided t-test, non-significant, P = 0.39; restraint male, 5.33 ± 1.33, n = 7 neurons, 2 mice; restraint female, 2.81 ± 0.39, n = 17 neurons, 2 mice; two-sided t-test, *P = 0.023; footshock male, 5.32 ± 0.89, n = 21 neurons, 2 mice; footshock female, 5.21 ± 0.92, n = 30 neurons, 2 mice; two-sided t-test, non-significant, P = 0.93. Data shown as mean ± s.e.m.
Supplementary Figure 4 SuperClomeleon reports GABAA receptor activity.
a. Schematic of the experimental design used for fiber photometry chloride imaging of NAc-projecting pPVT neurons. b. Representative image of SuperClomeleon expression in NAc-projecting neurons of the pPVT. c. Average SuperClomeleon response from NAc-projecting pPVT neurons in animals injected with the GABAA receptor agonist 4,5,6,7-tetrahydroisoxazolo(5,4-c) pyridin-3-ol (THIP, 8 mg/kg; green line) or saline (gray line; n = 3 mice). Data shown as mean ± s.e.m.
Supplementary Figure 5 Optical fiber placements for fiber photometry and optogenetic experiments.
a. Optical fiber placements for GCaMP6s experiment in Fig. 6a-d. b. Optical fiber placement for GCaMP6s experiment in Fig. 6e-h. c. Optical fiber placements for GCaMP6s experiment in Fig. 6i-l. d. Optical fiber placement for GCaMP6s experiment in Fig. 4h. e. Optical fiber placement for GCaMP6s experiment in Supplementary Fig. 8. f. Optical fiber placements for SuperClomeleon experiment in Fig. 1e-h. g. Optical fiber placement for SuperClomeleon experiment in Fig. 5a-d. h. Optical fiber placements for SuperClomeleon experiment in Fig. 5e-h. i. Optical fiber placement for SuperClomeleon experiment in Supplementary Fig. 3. j. Optical fiber placement for ChR2 experiment in Fig. 7. k. Optical fiber placement for halorhodopsin experiment in Fig. 6e-h. All circles depict the lowest position of the optical fibers for each subject.
Supplementary Figure 6 Robust expression of Drd2 and weak expression of Drd3 in the pPVT.
a. Fluorescent 2-plex in situ hybridization experiment showing the expression of Drd2 (red) and Drd3 (green) mRNA in the pPVT. b. Magnified view of the region depicted by the white square (a). 2-plex in situ hybridization experiments were independently repeated three times with samples collected from different subjects and similar results were obtained.
Supplementary Figure 7 Probe placements for microdialysis experiments.
a. Probe placements for microdialysis experiment in Fig. 4b. b. Representative image of hM4Di-mCherry expression in the LC of Dbh-Cre mice used for combined chemogenetic silencing of the LC and microdialysis of the PVT. Histological assessment of hM4Di-mCherry expression in the LC was independently repeated for each mouse included (seven total) and similar results were obtained. c. Probe placements for microdialysis experiment in saline treated mice (Fig. 4j). d. Probe placements for microdialysis experiment in CNO treated mice (Fig. 4j).
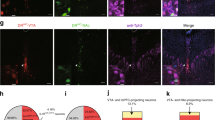
Supplementary Figure 8 TH+ afferents of the pPVT originate in the LC.
a. Representative image showing viral-assisted retrograde labelling of pPVT-projecting neurons in the ventral tegmental area (VTA). Antibody staining reveals TH-expressing neurons in VTA. Histological assessment of TH-expressing PVT-projecting neurons in the VTA was independently repeated for two mice and similar results were obtained. b. Magnified portion of the image shown in (a) depicts a single retrogradely-labelled neuron in VTA (arrow) that does not appear to be immunopositive for TH. c. Representative images from two separate experiments of viral-assisted retrograde labelling of pPVT-projecting neurons in the ventral tegmental area in LC. All neurons located within the LC displayed TH immunoreactivity. Histological assessment of TH-expressing PVT-projecting neurons in the LC was independently repeated for two mice and similar results were obtained.
Supplementary Figure 9 Stress-induced increase in extracellular NE in the pPVT is only partially dependent on the LC.
Schematic of the approach utilized for combined chemogenetic silencing of the LC and microdialysis of the pPVT (left). Summary plot depicting stress-induced increases in the extracellular concentration of NE following CNO (black) and saline vehicle (red) I.P. injection in mice expressing hM4Di in LC (n = 7 mice, per group). Data shown as mean ± s.e.m.
Supplementary Figure 10 Pharmacological blockade of the GABAA receptor increases the firing rate of neurons of the pPVT.
a. Sample traces showing the effect of the GABAA receptor blocker picrotoxin (PTX; 100 µM) on cell-attached recorded action potentials from two different pPVT neurons. b. Quantification of firing frequency in Hz (Baseline, 0.86 ± 0.23; PTX, 1.67 ± 0.41; n = 9 neurons, 3 mice, **P = 0.0082; two-sided Paired sample t-test). Data shown as mean ± s.e.m.
Supplementary Figure 11 Stress activates D2+ neurons of the pPVT.
a. Schematic of viral vector injections and optical fiber implantation for GCaMP6 fiber photometry experiments in Drd2-Cre mice. b. Representative image of GCaMP6s expression in D2+ neurons of the pPVT and optical fiber placement. Histological assessment of GCaMP6s expression in D2+ neurons of the pPVT was independently repeated for each mouse included (four total) and similar results were obtained. c. Average GCaMP6s response from D2+ pPVT neurons in animals subjected to footshock stress. Individual footshocks depicted by arrowheads. d. Average change in baseline fluorescence following footshock stress in %dF/F: Before, −0.68 ± 0.46; After, 6.71 ± 1.94; n = 4 mice; *P = 0.049; two-sided Paired sample t-test. Data shown as mean ± s.e.m.
Supplementary Figure 12 Most NAc-projecting neurons of the pPVT express D2 receptors.
a. Schematic of the stereotaxic injections for selectively expressing CTB in NAc-projecting neurons of the pPVT of D2-EGFP mice. b. Representative images showing coincident expression of D2-EGFP and CTB in the pPVT of wildtype mice (colocalization: 80% double-labelled/CTB, n = 4 mice).
Supplementary Figure 13 Tail suspension stress induces rapid activation of NAc-projecting neurons of the pPVT.
a. Schematic of the tail suspension protocol used during GCaMP6s imaging of NAc-projecting pPVT neurons. b. Representative trace showing the effect of brief tail suspensions (~10-12 s) on GCaMP6s fluorescence on a single subject. Arrowheads depict individual tail suspension events. c. Average GCaMP6s response from NAc-projecting pPVT neurons in animals subjected to tail suspension. Individual tail suspension events depicted by arrowheads. d. Average change in baseline fluorescence following tail suspension stress in %dF/F: Before, 0.24 ± 0.45; After, 9.88 ± 2.98; n = 4 mice; *P = 0.039; two-sided Paired sample t-test. Data shown as mean ± s.e.m.
Supplementary Figure 14 Changes in GCaMP6s fluorescence do not correlate with movement.
Correlation between the locomotion index (extracted from the FreezeFrame software, Actimetrics) and the GCaMP6s fluorescent signal (in %dF/F) for two individual subjects (n = 2 mice) from data shown in Fig. 6a-d. R-Square values are 0.008 (left) and 0.0004 (right).
Supplementary Figure 15 Optogenetic stimulation of LC–pPVT projections drives D2-receptor-mediated disinhibition in vitro.
a. Schematic of the experimental procedure employed to assess the effect of optogenetic stimulation of LC terminals onto NAc-projecting neurons of the pPVT. To elicit ChR2-evoked responses in the presence of TTX, 4-aminopyridine (4-AP, 100 µM) was added to the bath. b. Average plot depicting the effect of optogenetic stimulation of LC terminals with ChR2 on the average amplitude of mIPSC recorded from CTB-positive NAc-projecting neurons in the presence (% of Baseline, n = 9 neurons, 5 mice) or absence (% of Baseline, n = 8 neurons, 4 mice) of the D2-like blocker sulpiride (1 µM). c. Average plot depicting the effect of optogenetic stimulation of LC terminals with ChR2 on the average frequency of mIPSC recorded from CTB-positive NAc-projecting neurons in the presence (% of Baseline, n = 9 neurons, 5 mice) or absence (% of Baseline, n = 8 neurons, 4 mice) of the D2-like blocker sulpiride. Data shown as mean ± s.e.m.
Supplementary information
Rights and permissions
About this article
Cite this article
Beas, B.S., Wright, B.J., Skirzewski, M. et al. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat Neurosci 21, 963–973 (2018). https://doi.org/10.1038/s41593-018-0167-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0167-4
This article is cited by
-
A paraventricular thalamus to insular cortex glutamatergic projection gates “emotional” stress-induced binge eating in females
Neuropsychopharmacology (2023)
-
Dopamine receptors of the rodent fastigial nucleus support skilled reaching for goal-directed action
Brain Structure and Function (2023)
-
Sex differences in electrophysiological properties and voltage-gated ion channel expression in the paraventricular thalamic nucleus following repeated stress
Biology of Sex Differences (2022)
-
Phenethylamine is a substrate of monoamine oxidase B in the paraventricular thalamic nucleus
Scientific Reports (2022)
-
Reward and aversion processing by input-defined parallel nucleus accumbens circuits in mice
Nature Communications (2022)