Abstract
The mechanistic target of rapamycin complex 1 (mTORC1) has been reported to be necessary for metabotropic glutamate receptor-mediated long-term depression (mGluR-LTD). Here we found that mTORC1-deficient mice exhibit normal hippocampal mGluR-LTD and associated behaviors. Moreover, rapamycin blocks mGluR-LTD in mTORC1-deficient mice. However, both rapamycin and mGluR activation regulate mTOR complex 2 (mTORC2) activity, and mTORC2-deficient mice show impaired mGluR-LTD and associated behaviors. Thus, mTORC2 is a major regulator of mGluR-LTD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lüscher, C. & Huber, K. M. Neuron 65, 445–459 (2010).
Costa-Mattioli, M. & Monteggia, L. M. Nat. Neurosci. 16, 1537–1543 (2013).
Hoeffer, C. A. & Klann, E. Trends Neurosci. 33, 67–75 (2010).
Hou, L. & Klann, E. J. Neurosci. 24, 6352–6361 (2004).
Auerbach, B. D., Osterweil, E. K. & Bear, M. F. Nature 480, 63–68 (2011).
Laplante, M. & Sabatini, D. M. Cell 149, 274–293 (2012).
Wullschleger, S., Loewith, R. & Hall, M. N. Cell 124, 471–484 (2006).
Palmer, M. J., Irving, A. J., Seabrook, G. R., Jane, D. E. & Collingridge, G. L. Neuropharmacology 36, 1517–1532 (1997).
Benjamin, D., Colombi, M., Moroni, C. & Hall, M. N. Nat. Rev. Drug Discov. 10, 868–880 (2011).
Sarbassov, D. D. et al. Mol. Cell 22, 159–168 (2006).
Toschi, A. et al. Mol. Cell. Biol. 29, 1411–1420 (2009).
Huang, W. et al. Nat. Neurosci. 16, 441–448 (2013).
Goh, J. J. & Manahan-Vaughan, D. Cereb. Cortex 23, 1118–1125 (2013).
Goh, J. J. & Manahan-Vaughan, D. Hippocampus 23, 129–138 (2013).
Lamming, D. W. et al. Science 335, 1638–1643 (2012).
Huber, K. M., Kayser, M. S. & Bear, M. F. Science 288, 1254–1257 (2000).
Di Prisco, G. V. et al. Nat. Neurosci. 17, 1073–1082 (2014).
Graber, T. E. et al. Proc. Natl. Acad. Sci. USA 110, 16205–16210 (2013).
Stoica, L. et al. Proc. Natl. Acad. Sci. USA 108, 3791–3796 (2011).
Goorden, S. M. et al. Hum. Mol. Genet. 24, 3390–3398 (2015).
Dragatsis, I. & Zeitlin, S. Genesis. 26, 133–135 (2000).
Tsien, J. Z. et al. Cell 87, 1317–1326 (1996).
Zhu, P. J. et al. Cell 147, 1384–1396 (2011).
Ronesi, J. A. & Huber, K. M. J. Neurosci. 28, 543–547 (2008).
Buffington, S. A. et al. Cell 165, 1762–1775 (2016).
Acknowledgements
We thank K. Krnjevic, A. Placzek, and members of the Costa-Mattioli lab for comments on the manuscript. This work was supported by funds to M.C.-M. (NIMH 096816 and 112356, NINDS 076708, Department of Defense AR120254, Sammons Enterprises).
Author information
Authors and Affiliations
Contributions
P.J.Z., C.-J.C., J.M., and M.C.-M. designed the experiments and wrote the manuscript; P.J.Z. conducted electrophysiology, behavioral, and immunoblotting experiments and analyzed data. C.-J.C performed behavioral, immunohistochemistry, and immunoblotting experiments and analyzed data. J.M. conducted immunoblotting experiments and analyzed data. L.S. performed immunoblotting experiments and analyzed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated Supplementary Information
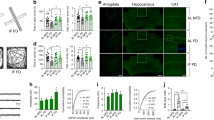
Supplementary Figure 1 mTORC1 activity is undetected in hippocampal CA1 neurons of Raptor fb-KO mice.
Green, immunohistochemistry staining for phosphorylated S6 (p-S6, Ser240/244). Red, immunohistochemistry staining for the neuronal marker NeuN. Experiments were repeated in two different cohorts of animals (two mice per genotype in each cohort) and samples were collected and processed separately.
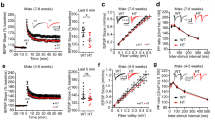
Supplementary Figure 2 Basal synaptic transmission did not differ between slices from control and Raptor fb-KO mice.
(a) Input-output plots show that fEPSPs as a function of presynaptic fiber volley was unaffected in Raptor fb-KO mice (n = 20) compared to control (n = 24) slices (linear regression; R2 = 0.64 for control and 0.63 for Raptor fb-KO mice. (b) Paired-pulse (PP) facilitation did not differ between control (n = 24) and Raptor fb-KO mice (n = 20), as determined by the plots of PP ratio (fEPSP2/fEPSP1) for various intervals of PP stimulation.
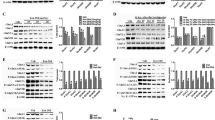
Supplementary Figure 3 High, but not low, concentrations of rapamycin blocks mGluR-LTD in control slices.
(a-b) Low concentrations of rapamycin (a, vehicle n = 7, 20 nM rapamycin n = 8, t13 = 1.592, P = 0.739; b, 200 nM rapamycin n = 8, vs vehicle t13 = 0.289, P = 1.0) had no effect on mGluR-LTD (rapamycin 20 nM vs 200 nM t13 = 1.348, P = 1.0). (c) mGluR-LTD is sensitive to high concentration rapamycin (1 μM rapamycin n = 8, vs vehicle t13 = 5.102, P < 0.001; rapamycin 20 nM vs rapamycin 1 μM, t14 = 3.633, P = 0.007; rapamycin 200 nM vs rapamycin 1 μM, t14 = 4.981, P < 0.001) (d-e) Western blot show that compared to vehicle treated slices, all concentrations of rapamycin reduce mTORC1 activity (p-S6) in control slices (20 nM rapamycin n = 7, vehicle n = 7, t12 = 5.843, P < 0.001; 200 nM rapamycin n = 7, vehicle n = 7, t12 = 13.262, P < 0.001; 1 μM rapamycin n = 6, vehicle n = 6, t10 = 10.116, P < 0.001). All data are presented as mean ± SEM. The statistics were based on two-sided Student’s t-test. Images of western blots were cropped to show only representative samples. Full-length blots can be found in supplementary Figure 8.
Supplementary Figure 4 High concentration of rapamycin (1 μM) had no effect on basal synaptic transmission.
Rapamycin (1 μM) did not affect fEPSPs as a function of presynaptic fiber volley (a), linear regression R2 = 0.643 for vehicle (n = 20) and 0.641 for rapamycin-treated (n = 16) slice or (b) paired-pulse (PP) facilitation. Data points are presented as mean ± SEM in (b).
Supplementary Figure 5 mTORC2 was activated by DHPG treatment and blocked by high concentration of rapamycin (1 μM).
(a-b) In control hippocampal slices, DHPG (100 μM, 10 min) triggers mTORC2 activity, as determined by increased phosphorylation of Akt at Ser-473 (n = 8 per group, P < 0.001, Mann-Whitney rank sum test). (c-d) In hippocampal slices, mTORC2 is sensitive to high (1 μM, n = 6 per group, t10 = 3.480, P = 0.006), but not low (20 nM, vehicle n = 7, rapamycin n = 7, t12 = 0.699, P = 0.498, or 200 nM, n = 7 per group, t12 = 0.570, P = 0.579), concentrations of rapamycin). Bar graph shows mean ± SEM of each group. The statistics were based on two-sided Student’s t-test. Images of western blots were cropped to show only representative samples. Full-length blots can be found in supplementary Figure 8.
Supplementary Figure 6 Normal locomotor and exploratory behavior in mTORC2-deficient mice.
(a-b) Control (n = 13) and Rictor fb-KO mice (n = 10) travelled similar distances during spatial recognition training (a, t21 = -1.307, P = 0.205) and re-exposure t22 = -0.687, P = 0.449) and spent similar time exploring identical objects during object recognition training (b, control n = 12, Left vs. Right, t22 = 0.988, P = 0.334; Rictor fb-KO mice n = 11, Left vs. Right, t20 = -0.847, P = 0.407). Box plots show the minimum, maximum, median, 25th, and 75th percentile of the groups. The mean values are indicated in thick lines. The statistics were based on two-sided Student’s t-test.
Supplementary Figure 7 Rapamycin (10 mg/kg) inhibits both spatial and object place learning.
(a) During re-exposure, rapamycin-treated mice (n = 10) spent significantly more time exploring the objects than vehicle-treated mice (n = 10, t18 = -5.089, P < 0.001). (b) Vehicle (n = 10) and rapamycin-treated (n = 10) mice travelled similar distances during spatial recognition training (t18 = 0.760, P = 0.457) and re-exposure (t18 = -1.589, P = 0.129). (c) Object recognition is impaired in rapamycin-treated mice (n = 9), since they show significantly less preference for novel objects compared to vehicle group (n = 9; t16 = 4.530, P < 0.001). (d) Rapamycin-treated and vehicle-treated mice spent similar time exploring identical objects during object recognition training (vehicle n = 9, Left vs. Right t16 = 1.167, P = 0.125; Rapamycin n = 9, Left vs. Right t16 = 1.060, P = 0.323). Box plots show the minimums, maximums, median, 25th, and 75th percentile of the groups. The mean values are indicated in thick lines. The statistics were based on two-sided Student’s t-test.
Supplementary Figure 8
Images of full-length blots presented in main and supplementary figures.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8
Rights and permissions
About this article
Cite this article
Zhu, P.J., Chen, CJ., Mays, J. et al. mTORC2, but not mTORC1, is required for hippocampal mGluR-LTD and associated behaviors. Nat Neurosci 21, 799–802 (2018). https://doi.org/10.1038/s41593-018-0156-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0156-7
This article is cited by
-
Targeted suppression of mTORC2 reduces seizures across models of epilepsy
Nature Communications (2023)
-
Organic cation transporter 2 contributes to SSRI antidepressant efficacy by controlling tryptophan availability in the brain
Translational Psychiatry (2023)
-
Perinatal exposure to pesticides alters synaptic plasticity signaling and induces behavioral deficits associated with neurodevelopmental disorders
Cell Biology and Toxicology (2023)
-
mTORC1 function in hippocampal parvalbumin interneurons: regulation of firing and long-term potentiation of intrinsic excitability but not long-term contextual fear memory and context discrimination
Molecular Brain (2022)
-
Raptor downregulation rescues neuronal phenotypes in mouse models of Tuberous Sclerosis Complex
Nature Communications (2022)