Abstract
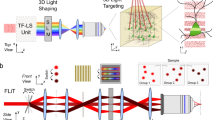
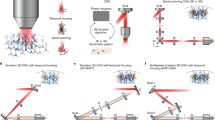
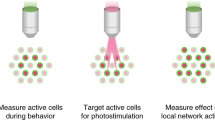
Understanding brain function requires technologies that can control the activity of large populations of neurons with high fidelity in space and time. We developed a multiphoton holographic approach to activate or suppress the activity of ensembles of cortical neurons with cellular resolution and sub-millisecond precision. Since existing opsins were inadequate, we engineered new soma-targeted (ST) optogenetic tools, ST-ChroME and IRES-ST-eGtACR1, optimized for multiphoton activation and suppression. Employing a three-dimensional all-optical read–write interface, we demonstrate the ability to simultaneously photostimulate up to 50 neurons distributed in three dimensions in a 550 × 550 × 100-µm3 volume of brain tissue. This approach allows the synthesis and editing of complex neural activity patterns needed to gain insight into the principles of neural codes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
London, M., Roth, A., Beeren, L., Häusser, M. & Latham, P. E. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature 466, 123–127 (2010).
Gollisch, T. & Meister, M. Rapid neural coding in the retina with relative spike latencies. Science 319, 1108–1111 (2008).
Histed, M. H. & Maunsell, J. H. R. Cortical neural populations can guide behavior by integrating inputs linearly, independent of synchrony. Proc. Natl Acad. Sci. USA 111, E178–E187 (2014).
Bruno, R. M. & Sakmann, B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312, 1622–1627 (2006).
Harris, K. D. & Mrsic-Flogel, T. D. Cortical connectivity and sensory coding. Nature 503, 51–58 (2013).
Panzeri, S., Harvey, C. D., Piasini, E., Latham, P. E. & Fellin, T. Cracking the neural code for sensory perception by combining statistics, intervention, and behavior. Neuron 93, 491–507 (2017).
Jepson, L. H. et al. High-fidelity reproduction of spatiotemporal visual signals for retinal prosthesis. Neuron 83, 87–92 (2014).
Clancy, K. B., Schnepel, P., Rao, A. T. & Feldman, D. E. Structure of a single whisker representation in layer 2 of mouse somatosensory cortex. J. Neurosci. 35, 3946–3958 (2015).
Ohki, K., Chung, S., Ch’ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Rickgauer, J. P. & Tank, D. W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl Acad. Sci. USA 106, 15025–15030 (2009).
Vaziri, A. & Emiliani, V. Reshaping the optical dimension in optogenetics. Curr. Opin. Neurobiol. 22, 128–137 (2012).
Packer, A. M. et al. Two-photon optogenetics of dendritic spines and neural circuits. Nat. Methods 9, 1202–1205 (2012).
Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods 9, 1171–1179 (2012).
Packer, A. M., Russell, L. E., Dalgleish, H. W. P. & Häusser, M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat. Methods 12, 140–146 (2015).
Rickgauer, J. P., Deisseroth, K. & Tank, D. W. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat. Neurosci. 17, 1816–1824 (2014).
Carrillo-Reid, L., Yang, W., Bando, Y., Peterka, D. S. & Yuste, R. Imprinting and recalling cortical ensembles. Science 353, 691–694 (2016).
Emiliani, V., Cohen, A. E., Deisseroth, K. & Häusser, M. All-optical interrogation of neural circuits. J. Neurosci. 35, 13917–13926 (2015).
Dal Maschio, M., Donovan, J. C., Helmbrecht, T. O. & Baier, H. Linking neurons to network function and behavior by two-photon holographic optogenetics and volumetric imaging. Neuron 94, 774–789.e5 (2017).
Papagiakoumou, E. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010).
Forli, A. et al. Two-photon bidirectional control and imaging of neuronal excitability with high spatial resolution in vivo. Cell Rep. 22, 3087–3098 (2018).
Nikolenko, V. et al. SLM microscopy: scanless two-photon imaging and photostimulation with spatial light modulators. Front. Neural Circuits 2, 5 (2008).
Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008).
Emiliani, V. et al. Wave front engineering for microscopy of living cells. Opt. Express 13, 1395–1405 (2005).
Ronzitti, E. et al. Submillisecond optogenetic control of neuronal firing by two-photon holographic photoactivation of Chronos. J. Neurosci. 37, 10679–10689 (2017).
Shemesh, O. A. et al. Temporally precise single-cell-resolution optogenetics. Nat. Neurosci. 20, 1796–1806 (2017).
Hernandez, O. et al. Three-dimensional spatiotemporal focusing of holographic patterns. Nat. Commun. 7, 11928 (2016).
Pégard, N. C. et al. Three-dimensional scanless holographic optogenetics with temporal focusing (3D-SHOT). Nat. Commun. 8, 1228 (2017).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Govorunova, E. G., Sineshchekov, O. A., Janz, R., Liu, X. & Spudich, J. L. Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics. Science 349, 647–650 (2015).
Ahmadian, Y., Packer, A. M., Yuste, R. & Paninski, L. Designing optimal stimuli to control neuronal spike timing. J. Neurophysiol. 106, 1038–1053 (2011).
Baker, C. A., Elyada, Y. M., Parra, A. & Bolton, M. M. L. Cellular resolution circuit mapping with temporal-focused excitation of soma-targeted channelrhodopsin. eLife 5, 1–15 (2016).
Wu, C., Ivanova, E., Zhang, Y. & Pan, Z. H. rAAV-mediated subcellular targeting of optogenetic tools in retinal ganglion cells in vivo. PLoS One 8, e66332 (2013).
Podgorski, K. & Ranganathan, G. Brain heating induced by near-infrared lasers during multiphoton microscopy. J. Neurophysiol. 116, 1012–1023 (2016).
Kato, H. E. et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482, 369–374 (2012).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Chow, B. Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Govorunova, E. G., Sineshchekov, O. A. & Spudich, J. L. Proteomonas sulcata ACR1: a fast anion channelrhodopsin. Photochem. Photobiol. 92, 257–263 (2016).
Berndt, A. et al. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc. Natl Acad. Sci. USA 113, 822–829 (2016).
Markram, H. et al. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 (2004).
Taniguchi, H. et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013 (2011).
Hippenmeyer, S. et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005).
Wekselblatt, J. B., Flister, E. D., Piscopo, D. M. & Niell, C. M. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 115, 2852–2866 (2016).
Grewe, B. F., Voigt, F. F., van ’t Hoff, M. & Helmchen, F. Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens. Biomed. Opt. Express 2, 2035–2046 (2011).
Yang, W., Carrillo-Reid, L., Bando, Y., Peterka, D. S. & Yuste, R. Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. eLife 7, e32671 (2018).
Adesnik, H. & Scanziani, M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature 464, 1155–1160 (2010).
Harris, K. D. & Thiele, A. Cortical state and attention. Nat. Rev. Neurosci. 12, 509–523 (2011).
Churchland, A. K. et al. Variance as a signature of neural computations during decision making. Neuron 69, 818–831 (2011).
Andrasfalvy, B. K., Zemelman, B. V., Tang, J. & Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl Acad. Sci. USA 107, 11981–11986 (2010).
Huber, D. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64 (2008).
Lim, S. T., Antonucci, D. E., Scannevin, R. H. & Trimmer, J. S. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron 25, 385–397 (2000).
Gerchberg, R. W. & Saxton, W. O. A practical algorithm for the determination of phase from image and diffraction plane pictures. Optik (Stuttg.) 35, 237–246 (1972).
Zhang, J., Pégard, N., Zhong, J. & Waller, L. 3D computer generated holograms by nonconvex optimization. Optica 4, 1306–1313 (2017).
Pluta, S. et al. A direct translaminar inhibitory circuit tunes cortical output. Nat. Neurosci. 18, 1631–1640 (2015).
Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at bioRxiv https://doi.org/10.1101/061507 (2016).
Acknowledgements
We thank M. Feller, A. Naka, and J. Brown for critical feedback on the manuscript and discussions. We thank C. Baker and M. Bolton for soma-targeted ChR2 AAVs. We thank M. Li and the UC Berkeley Vision Science Core, Gene Delivery Module, for preparation of AAVs (supported by NIH Core Grant P30 EY003176). We deeply appreciate the efforts of D. Chu, C. Douglas, and R. Hakim for important technical assistance. We thank D. Taylor for help with mouse work and histology. H.A. is a New York Stem Cell Foundation-Robertson Investigator. This work was supported by The New York Stem Cell Foundation and by grants from the Arnold and Mabel Beckman Foundation, NINDS grant DP2NS087725-01, the McKnight Foundation, NINDS award F32NS095690-01 to A.R.M., the Simon’s Foundation Collaboration for the Global Brain award 415569 to I.A.O., and a fellowship from the David and Lucille Packard Foundation to L.W. This research was developed with funding from the Defense Advanced Research Projects Agency (DARPA), Contract No. N660011-17-C-4015.
Author information
Authors and Affiliations
Contributions
A.R.M., I.A.O., N.C.P. and H.A conceived the project and built the system. A.R.M. designed and performed all experiments involving excitatory opsins. I.A.O. designed and performed all experiments involving inhibitory opsins. N.C.P. designed and assembled the light paths and wrote custom holography software. S.S. performed cloning, mutagenesis, cell culture, and one-photon recordings in CHO cells. A.R.M., I.A.O., N.C.P., and E.H.L. wrote code and developed software for experimental control. K.C. performed histology. S.G.B. performed modeling of the Chronos pore region. L.W. contributed expertise on holographic design. A.R,M., I.A.O., N.C.P., and H.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–23
Supplementary Table 1 – Optical Setup References
This table provides details on each optical stimulation path used in this study and provides a quick reference to determine which experiments were performed using each setup
Supplementary Table 2 – 3D-SHOT 2.0
This table provides technical details and a calculation tool to allow readers to set up a 3D-SHOT stimulation path
Supplementary Video 1 – SLM performance limitations for high speed 3D-SHOT
This video demonstrates high speed switching of holograms at 300 Hz
Supplementary Video 2 – All-Optical Ensemble Stimulation in 3D
Average dF/F movies of ensembles stimulation data at 30 Hz as in Fig7-8
Rights and permissions
About this article
Cite this article
Mardinly, A.R., Oldenburg, I.A., Pégard, N.C. et al. Precise multimodal optical control of neural ensemble activity. Nat Neurosci 21, 881–893 (2018). https://doi.org/10.1038/s41593-018-0139-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0139-8
This article is cited by
-
The logic of recurrent circuits in the primary visual cortex
Nature Neuroscience (2024)
-
The influence of cortical activity on perception depends on behavioral state and sensory context
Nature Communications (2024)
-
Ultrafast light targeting for high-throughput precise control of neuronal networks
Nature Communications (2023)
-
A distributed and efficient population code of mixed selectivity neurons for flexible navigation decisions
Nature Communications (2023)
-
The future of brain–machine interfaces is optical
Nature Electronics (2023)