Abstract
Social animals detect the affective states of conspecifics and utilize this information to orchestrate social interactions. In a social affective preference text in which experimental adult male rats could interact with either naive or stressed conspecifics, the experimental rats either approached or avoided the stressed conspecific, depending upon the age of the conspecific. Specifically, experimental rats approached stressed juveniles but avoided stressed adults. Inhibition of insular cortex, which is implicated in social cognition, and blockade of insular oxytocin receptors disrupted the social affective behaviors. Oxytocin application increased intrinsic excitability and synaptic efficacy in acute insular cortex slices, and insular oxytocin administration recapitulated the behaviors observed toward stressed conspecifics. Network analysis of c-Fos immunoreactivity in 29 regions identified functional connectivity between insular cortex, prefrontal cortex, amygdala and the social decision-making network. These results implicate insular cortex as a key component in the circuit underlying age-dependent social responses to stressed conspecifics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Darwin, C. The Expression of the Emotions in Man and Animals. (St. Martin’s Press, London, New York, 1976). Julian Friedmann.
O’Connell, L. A. & Hofmann, H. A. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639 (2011).
Gu, X. et al. Anterior insular cortex is necessary for empathetic pain perception. Brain 135, 2726–2735 (2012).
Ibañez, A., Gleichgerrcht, E. & Manes, F. Clinical effects of insular damage in humans. Brain Struct. Funct. 214, 397–410 (2010).
Leigh, R. et al. Acute lesions that impair affective empathy. Brain 136, 2539–2549 (2013).
Blanken, L. M. et al. Cortical morphology in 6- to 10-year old children with autistic traits: a population-based neuroimaging study. Am. J. Psychiatry 172, 479–486 (2015).
Di Martino, A. et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am. J. Psychiatry 166, 891–899 (2009).
Morita, T. et al. Emotional responses associated with self-face processing in individuals with autism spectrum disorders: an fMRI study. Soc. Neurosci. 7, 223–239 (2012).
Odriozola, P. et al. Insula response and connectivity during social and non-social attention in children with autism. Soc. Cogn. Affect. Neurosci. 11, 433–444 (2016).
Gogolla, N. The insular cortex. Curr. Biol. 27, R580–R586 (2017).
Allen, G. V. & Cechetto, D. F. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. II. Ascending projections. J. Comp. Neurol. 330, 421–438 (1993).
Ohara, P. T. et al. Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat. J. Neurocytol. 32, 131–141 (2003).
Shi, C. J. & Cassell, M. D. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J. Comp. Neurol. 399, 440–468 (1998).
Sato, F. et al. Projections from the insular cortex to pain-receptive trigeminal caudal subnucleus (medullary dorsal horn) and other lower brainstem areas in rats. Neuroscience 233, 9–27 (2013).
Allen, G. V., Saper, C. B., Hurley, K. M. & Cechetto, D. F. Organization of visceral and limbic connections in the insular cortex of the rat. J. Comp. Neurol. 311, 1–16 (1991).
Yasui, Y., Breder, C. D., Saper, C. B. & Cechetto, D. F. Autonomic responses and efferent pathways from the insular cortex in the rat. J. Comp. Neurol. 303, 355–374 (1991).
Knobloch, H. S. et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566 (2012).
Dumais, K. M., Bredewold, R., Mayer, T. E. & Veenema, A. H. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm. Behav. 64, 693–701 (2013).
Donaldson, Z. R. & Young, L. J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904 (2008).
Shamay-Tsoory, S. G. & Abu-Akel, A. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202 (2016).
Burkett, J. P. et al. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378 (2016).
Aoki, Y. et al. Oxytocin improves behavioural and neural deficits in inferring others’ social emotions in autism. Brain 137, 3073–3086 (2014).
Scheele, D. et al. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 39, 2078–2085 (2014).
de Waal, F. B. M. & Preston, S. D. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 18, 498–509 (2017).
Silberberg, A. et al. Desire for social contact, not empathy, may explain “rescue” behavior in rats. Anim. Cogn. 17, 609–618 (2014).
Bullmore, E. & Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009).
Staub, E. Helping a distressed person: social, personality, and stimulus determinants. Adv. Exp. Soc. Psychol.. 7, 293–341 (1974).
Spear, L. P. Adolescent alcohol exposure: are there separable vulnerable periods within adolescence? Physiol. Behav. 148, 122–130 (2015).
Brudzynski, S. M. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr. Opin. Neurobiol. 23, 310–317 (2013).
Marlin, B. J., Mitre, M., D’amour, J. A., Chao, M. V. & Froemke, R. C. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015).
Gimpl, G. & Fahrenholz, F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 81, 629–683 (2001).
Power, J. D., Schlaggar, B. L., Lessov-Schlaggar, C. N. & Petersen, S. E. Evidence for hubs in human functional brain networks. Neuron 79, 798–813 (2013).
Mikosz, M., Nowak, A., Werka, T. & Knapska, E. Sex differences in social modulation of learning in rats. Sci. Rep. 5, 18114 (2015).
Choe, H. K. et al. Oxytocin mediates entrainment of sensory stimuli to social cues of opposing valence. Neuron 87, 152–163 (2015).
Insel, T. R. & Fernald, R. D. How the brain processes social information: searching for the social brain. Annu. Rev. Neurosci. 27, 697–722 (2004).
Valenta, J. G. & Rigby, M. K. Discrimination of the odor of stressed rats. Science 161, 599–601 (1968).
Sotocinal, S. G. et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 7, 55 (2011).
Kiyokawa, Y. Social odors: alarm pheromones and social buffering. Curr. Top. Behav. Neurosci. 30, 47–65 (2017).
Burgdorf, J. et al. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J. Comp. Psychol. 122, 357–367 (2008).
Oettl, L.L. & Kelsch, W. Oxytocin and olfaction. Curr. Top. Behav. Neurosci. (2017).
Langford, D. J. et al. Social approach to pain in laboratory mice. Soc. Neurosci. 5, 163–170 (2010).
Ben-Ami Bartal, I., Rodgers, D. A., Bernardez Sarria, M. S., Decety, J. & Mason, P. Pro-social behavior in rats is modulated by social experience. eLife 3, e01385 (2014).
de Waal, F. B. Putting the altruism back into altruism: the evolution of empathy. Annu. Rev. Psychol. 59, 279–300 (2008).
Moaddab, M., Hyland, B. I. & Brown, C. H. Oxytocin excites nucleus accumbens shell neurons in vivo. Mol. Cell. Neurosci. 68, 323–330 (2015).
Adolphs, R. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716 (2009).
Bartz, J. A., Zaki, J., Bolger, N. & Ochsner, K. N. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15, 301–309 (2011).
Decety, J. & Moriguchi, Y. The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. Biopsychosoc. Med.. 1, 22 (2007).
Thioux, M. & Keysers, C. Empathy: shared circuits and their dysfunctions. Dialog-. Clin. Neurosci. 12, 546–552 (2010).
LoParo, D. & Waldman, I. D. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol. Psychiatry 20, 640–646 (2015).
Skuse, D. H. et al. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc. Natl. Acad. Sci. USA 111, 1987–1992 (2014).
Paxinos, G. & Watson, C. The Rat Brain Atlas in Stereotaxic Coordinates. 4th edition, (Academic Press, San Diego, CA, USA, 1998).
Smith, C. J., Wilkins, K. B., Mogavero, J. N. & Veenema, A. H. Social novelty investigation in the juvenile rat: modulation by the μ-opioid system. J. Neuroendocrinol. 27, 752–764 (2015).
Christianson, J. P. et al. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol. Psychiatry 70, 458–464 (2011).
Manning, M. et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 24, 609–628 (2012).
Lukas, M., Toth, I., Veenema, A. H. & Neumann, I. D. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology 38, 916–926 (2013).
Tye, K. M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Sidiropoulou, K. et al. Dopamine modulates an mGluR5-mediated depolarization underlying prefrontal persistent activity. Nat. Neurosci. 12, 190–199 (2009).
Dölen, G., Darvishzadeh, A., Huang, K. W. & Malenka, R. C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013).
Szot, P., Bale, T. L. & Dorsa, D. M. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Brain Res. Mol. Brain Res. 24, 1–10 (1994).
Dumais, K. M. & Veenema, A. H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 40, 1–23 (2016).
Varela, J. A., Wang, J., Christianson, J. P., Maier, S. F. & Cooper, D. C. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. J. Neurosci. 32, 12848–12853 (2012).
Turrigiano, G. G., Leslie, K. R., Desai, N. S., Rutherford, L. C. & Nelson, S. B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896 (1998).
Gschwendt, M. et al. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett.. 392, 77–80 (1996).
Christianson, J. P. et al. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J. Neurosci. 28, 13703–13711 (2008).
Vetere, G. et al. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron 94, 363–374.e4 (2017).
Wheeler, A. L. et al. Identification of a functional connectome for long-term fear memory in mice. PLOS Comput. Biol. 9, e1002853 (2013).
Tanimizu, T. et al. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J. Neurosci. 37, 4103–4116 (2017).
Teles, M. C., Almeida, O., Lopes, J. S. & Oliveira, R. F. Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc. Biol. Sci.. 282, 20151099 (2015).
Newman, M. E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 103, 8577–8582 (2006).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag, New York, 2009).
Acknowledgements
The authors thank M. Manning (University of Toledo) for providing OTRa, K. Deisseroth (Stanford University) for making optogenetic vectors freely available, and A. Veenema and D. Adams for discussions related to the project. Funding for this work was provided by the Boston College Undergraduate Research Fellowships; National Science Foundation Grant #1258923 to M.M.R.-C; NIH grants MH103401 to M.R. and MH093412 and MH109545 to J.P.C.; and Brain and Behavior Research Foundation grant No. 19417 to J.P.C.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.P.C., J.A.V., M.M.R.-C., M.R. Methodology: M.M.R.-C., J.A.V., M.R. J.P.C. Investigation: J.A.V., M.M.R.-C., K.B.G., A.P., M.M., M.R., J.P.C. Writing, original draft: J.A.V., M.M.R.-C., and J.P.C.;;revising & editing: M.M.R.-C., M.R., J.P.C. Funding acquisition, J.P.C., M.M.R.-C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Conspecific behavior in social affective preference test.
(a) An observer blind to conspecific treatment tallied the time spent inactive, self-grooming, engaged in social interaction with the experimental adult and time spent sniffing and exploring the conspecific chamber of 12 pairs of target rats from each of the PN 30 and PN 50 treatment groups. The average portion (% of total) time spent in each behavior during the 5-minute test is shown for each treatment. The analyses indicate that the conspecifics spent the majority of time in active behaviors, namely sniffing and exploring the chamber. (b) Mean (+/− SEM) time spent self-grooming. Separate t-tests were conducted to compare time in each behavior and revealed a significant increase in self grooming after stress in the PN 30 group. *t(22) = 2.47, p = 0.022 (2-tailed). No other comparisons reached significance, and self grooming did not correlate with behavior of the experimental adult rats.
Supplementary Figure 2 Repeated one-on-one testing and SAP tests in females.
(a) Adult male rats (n = 6) were given a series of 4, 1-on-1 social interaction tests (5 min duration) in each of the following conditions: naive-juvenile, naive-adult, stress-juvenile and stress-adult; test order was counter balanced using a Latin square design. Tests were exactly as described in the Online Methods and separated by 20 to 30 min. All conspecific stimuli were novel/unfamiliar pairs. Two way repeated measures ANOVA revealed significant interaction of age and stress, F(1, 5) = 20.549, p = 0.006, with significant differences between Naive and Stress at both ages, *p = 0.022 (2-tailed), #p = 0.028 (1-tailed). (b) Mean (+/− SEM, n = 6) percent preference for stressed conspecific. This series of tests in (a) was repeated for 3 consecutive days and data were converted to percent preference for the stressed conspecific by age, and by day. PN30 and PN50 preference scores were significantly different on all three days. ANOVA revealed significant Age & Day interaction F(2, 10) = 5.211, p = 0.028, ****p < 0.0001, **p < 0.007 (Sidak). (c) Mean social interaction time. SAP tests were performed on adult female, regularly cycling rats with pairs of male juvenile or adult conspecifics as interaction stimuli (N = 8 females). SAP tests were exactly as described in the online methods. A significant 2-way interaction, F(1, 14) = 15.52, p = 0.002 indicated that the females also exhibit preference to interact with stressed juvenile conspecifics but avoid stressed adults, *p < 0.05 (Sidak). (d) Results in c. shown as Mean % time interacting with stressed conspecific for comparison, **t(7) = 3.51, p = 0.009 (2-tailed).
Supplementary Figure 3 Ultrasonic vocalizations and social interaction.
Extended analysis of data described in Fig. 1i–k. Peri-event histograms showing frequency of Rising (A) and Trill (B) USVs in the different conspecific treatment conditions (NJ, n = 10, NA, n = 10, SJ, n = 11, SA, n = 11). Time 0 is equal to the beginning of a social interaction bout. Bins are 250 ms. Calls were included from all rats in the treatment condition for social interaction bouts 3 s or longer. (C) Mean (+/− S.E.M.) rate of vocalization (rising and trills combined) 2.5 s before and 2.5 s after the beginning of a 3 s (or longer) social interaction bout. Values normalized to a pre-bout baseline 5 s prior to the social interaction. (D) Mean (+ S.E.M.) percent of total USVs detected during 5 minute social interaction bout that occurred during social interactions. Stress reduced within-bout vocalizations in the adult condition (Main effect of Age, F(3, 74) = 8.523, p < 0.0001). *SA vs. NJ, p = 0.019; SA vs. NA, p = 0.016; SA vs. SJ, p = 0.0005 (Fisher LSD). NJ = naive juvenile, NA = naive adult, SJ = stressed juvenile, SA = stressed adult.
Supplementary Figure 4 Inhibition of insular cortex with muscimol reverses SAP test behavior.
In a pilot study, rats were implanted with insular cortex cannula for microinjection exactly as described in the online methods. After 7 days of recovery rats received SAP tests with either PN 30 (n = 9) or PN 50 (n = 10) conspecifics. SAP tests were as in Fig. 2: Habituation to arena on Day 1, Habituation with exposure to naïve conspecifics on Day 2, and SAP tests with one conspecific exposed to 2 footshocks prior to test on Day 3. Either Vehicle (0.9% saline) or muscimol (100 ng/side in saline) was injected to the insular cortex 60 minutes prior to SAP tests on day 3. This experiment was different in design from the others included in the main text because rats received only 1 SAP test precluding the more powerful within-subject comparisons of drug effect. SAP behavior is shown as the mean (+/− SEM) percent of time spent interacting with the stressed conspecific of the total time spent in social interaction during the SAP test. A 2-way between subjects ANOVA revealed a significant Age by Drug interaction, F(1, 34) = 16.27, p < 0.001. Post hoc comparisons between drug conditions within each age revealed a significant decrease in time spent interacting with the stressed PN 30 juvenile conspecific by muscimol (**p = 0.005, Sidak) and a significant increase in time spent interacting with the stressed PN 50 adult conspecific (*p = 0.043, Sidak).
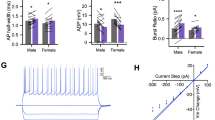
Supplementary Figure 5 Verification of halorhodopsin transductions and behavioral analysis in SAP tests.
Placement of AAV5-CamKIIa-eNpHr3.0-mCherry transfections in experimental adult rats given SAP tests with (a) PN 30 juvenile conspecifics or (b) PN 50 conspecifics. Different colored traces correspond to individual replicates. In each case the tip of the fiber optic cannula was found within the insular cortex and either directly above or within the area marked as containing mCherry (unamplified) fluorescence. (c) Viral transfer was validated in whole cell recordings of mCherry (above DIC image, below mCherry false colored yellow. 40x, Scale bar = 40 μm) positive neurons in acute slices containing the insular cortex. Application of green light (wavelength = 532 nm, 10 mW/mm2) through the objective of the electrophysiology microscope induced robust hyperpolarizations which silenced spiking when provided during a train of evoked spikes, scale bar 50 mV/250 ms. (d) Mean (+/− SEM) spikes during sequence of OFF/ON/OFF application as in panel (D), n = 6 cells, one-way repeated measures ANOVA, F(2, 15) = 22.82, p < 0.0001 with the spike rate in the ON condition significantly less than either off condition, ***p < 0.0001 (Sidak). (e) Mean (+SEM) time spent in different behaviors of adult conspecifics in optogenetic experiment (PN30, n = 9, PN50, n = 11). Behavior levels are equal between light ON and OFF conditions except for increased immobility in the PN50 light ON condition, Age by Light by Behavior interaction, F(4, 60) = 3.01, p = 0.025. *p = 0.001 (Sidak). The increase in immobility is likely due to 2 of 11 rats that exhibited very high immobility (25–26 s). Brain Atlas illustrations were reproduced with permission as previously published in The Brain Atlas in Stereotaxic Coordinates, 4th Edition, Paxinos, G. & Watson, C. Pages 296, 303, 306–317 & 332. Copyright Elsevier (1998).
Supplementary Figure 6 Placement of cannula tips for microinjection experiments and excluded subject data.
(a) Cannula tip placement within the insular cortex was verified in 40 μm fresh frozen sections stained with Cresyl Violet under light microscopy. Tip locations are indicated. Section distance from Bregma indicated in mm. Open/White Circles relate to Fig. 5b-c, Red circles relate to Fig. 5e and Gold circles to Fig. 5f, Grey circles related to Fig. 5g-h. (b) SAP test behavior from the rats excluded from mechanistic experiments because the baseline behavior (light OFF or vehicle condition) did not reflect the phenomena under study. LEFT: excluded from Fig. 2f-g, CENTER: excluded from Fig. 5b-c, RIGHT: excluded from Fig. 5g-h. Brain Atlas illustrations were reproduced with permission as previously published in The Brain Atlas in Stereotaxic Coordinates, 4th Edition, Paxinos, G. & Watson, C. Pages 296, 303, 306–317 & 332. Copyright Elsevier (1998).
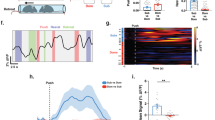
Supplementary Figure 7 Representative examples of c-Fos immunohistochemistry and regions of interest.
Coronal diagrams on left correspond to regions of interest indicated within the representative digital photomicrographs on the right. Images were acquired and analyzed with equal exposure and analysis was conducted on images without adjustments to brightness or contrast. The representative images have been adjusted to facilitate presentation. This experiment was replicated 11 times per group. List of abbreviations is provided in Supplementary Figure 8. Scale bars = 200 μm. Brain Atlas illustrations were reproduced with permission as previously published in The Brain Atlas in Stereotaxic Coordinates, 4th Edition, Paxinos, G. & Watson, C. Pages 296, 303, 306–317 & 332. Copyright Elsevier (1998).
Supplementary Figure 8 Extended analysis of Fos network.
(A) Correlation matrices (Kendall’s tau) organized as in Fig. 6 by conspecific age and stress condition (n = 11/group, N = 44 for All condition). (B) Correlations (Box plots range = 25th to 75th percentile, line indicates median) within and between modules identified by a community detection analysis (described in Fig. 6b). (C) Effect of stress on participation coefficient computed as in Fig. 6 (ALL condition) and for each age and stress condition. NJ = naive juvenile, NA = naive adult, SJ = stressed juvenile, SA = stressed adult. See Sup. Table 1 for abbreviation key.
Supplementary Figure 9 Manipulations of insular cortex reverse SAP test behavior.
From visual inspection of the results of the optogenetic and pharmacology experiments it appeared that manipulating the insular cortex not only prevented the expression of preference behavior in the SAP test, but that it might actually switch preference in the opposite direction. To test this possibility, % time interacting with the stressed conspecific scores were pooled from all of the mechanistic experiments (halorhodopsin, OTRa, PKC inhibitor, n = 26 for PN30, n = 25 for PN50) and we conducted one-sample t-tests (2-tailed) comparing each condition to 50% (no preference). Indeed, disrupting insular function reversed SAP behavior. In PN30 rats, the mean (+SEM) % time interacting with the stressed conspecific switched away from the stressed juvenile, t(25) = 3.35, **p = 0.003 and in PN 50 rats it switched toward interacting with the stressed adult, t(24) = 2.30, *p = 0.013.
Supplementary Figure 10 Intra-insula oxytocin receptor antagonist (OTRa) does not alter social interaction.
Adult male rats were implanted with insular cortex cannula and given 3 minute social interaction tests with naive, juvenile conspecifics (N = 16). Vehicle or OTRa (as described in Online Methods) was injected 15 min before testing on day 1 or day 2. All rats were tested under both drug conditions with test order counterbalanced. There was no effect of OTRa on social interaction t(15) = 1.61, p = 0.13 (2-tailed).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10
Videos
Supplementary Video 1 - Social Affective Preference Test.
In this example, the experimental adult was presented with a stressed PN 30 conspecific in the left chamber and a naïve PN 30 conspecific on the right. The appearance of a green rectangle identifies bouts of social interaction with the stressed conspecific. Video length = 39 seconds
Rights and permissions
About this article
Cite this article
Rogers-Carter, M.M., Varela, J.A., Gribbons, K.B. et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci 21, 404–414 (2018). https://doi.org/10.1038/s41593-018-0071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-018-0071-y
This article is cited by
-
A novel micro-ECoG recording method for recording multisensory neural activity from the parietal to temporal cortices in mice
Molecular Brain (2023)
-
Detection, processing and reinforcement of social cues: regulation by the oxytocin system
Nature Reviews Neuroscience (2023)
-
Social circuits and their dysfunction in autism spectrum disorder
Molecular Psychiatry (2023)
-
The anterior insular cortex processes social recognition memory
Scientific Reports (2023)
-
Neural circuits regulating prosocial behaviors
Neuropsychopharmacology (2023)