Abstract
Autism spectrum disorder (ASD) is characterized by impaired social communication, often attributed to misreading of emotional cues. Why individuals with ASD misread emotions remains unclear. Given that terrestrial mammals rely on their sense of smell to read conspecific emotions, we hypothesized that misreading of emotional cues in ASD partially reflects altered social chemosignaling. We found no difference between typically developed (TD) and cognitively able adults with ASD at explicit detection and perception of social chemosignals. Nevertheless, TD and ASD participants dissociated in their responses to subliminal presentation of these same compounds: the undetected ‘smell of fear’ (skydiver sweat) increased physiological arousal and reduced explicit and implicit measures of trust in TD but acted opposite in ASD participants. Moreover, two different undetected synthetic putative social chemosignals increased or decreased arousal in TD but acted opposite in ASD participants. These results implicate social chemosignaling as a sensory substrate of social impairment in ASD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lübke, K. T. & Pause, B. M. Always follow your nose: the functional significance of social chemosignals in human reproduction and survival. Horm. Behav. 68, 134–144 (2015).
McClintock, M. K. Reproduction in Context (MIT Press, Cambridge, MA, USA, 2000).
de Groot, J. H., Smeets, M. A., Kaldewaij, A., Duijndam, M. J. & Semin, G. R. Chemosignals communicate human emotions. Psychol. Sci. 23, 1417–1424 (2012).
Wysocki, C. J. & Preti, G. Facts, fallacies, fears, and frustrations with human pheromones. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 281, 1201–1211 (2004).
Gelstein, S. et al. Human tears contain a chemosignal. Science 331, 226–230 (2011).
Frumin, I. et al. A social chemosignaling function for human handshaking. eLife 4, e05154 (2015).
Mitro, S., Gordon, A. R., Olsson, M. J. & Lundström, J. N. The smell of age: perception and discrimination of body odors of different ages. PLoS One 7, e38110 (2012).
Olsson, M. J. et al. The scent of disease: human body odor contains an early chemosensory cue of sickness. Psychol. Sci. 25, 817–823 (2014).
Mutic, S., Brünner, Y. F., Rodriguez-Raecke, R., Wiesmann, M. & Freiherr, J. Chemosensory danger detection in the human brain: body odor communicating aggression modulates limbic system activation. Neuropsychologia 99, 187–198 (2017).
de Groot, J. H. et al. A sniff of happiness. Psychol. Sci. 26, 684–700 (2015).
de Groot, J. H. B. & Smeets, M. A. M. Human fear chemosignaling: evidence from a meta-analysis. Chem. Senses 42, 663–673 (2017).
Zhou, W. & Chen, D. Fear-related chemosignals modulate recognition of fear in ambiguous facial expressions. Psychol. Sci. 20, 177–183 (2009).
Chen, D., Katdare, A. & Lucas, N. Chemosignals of fear enhance cognitive performance in humans. Chem. Senses 31, 415–423 (2006).
Wudarczyk, O. A. et al. Chemosensory anxiety cues enhance the perception of fearful faces - an fMRI study. Neuroimage 143, 214–222 (2016).
Sobel, N. et al. Blind smell: brain activation induced by an undetected air-borne chemical. Brain 122, 209–217 (1999).
Mujica-Parodi, L. R. et al. Chemosensory cues to conspecific emotional stress activate amygdala in humans. PLoS One 4, e6415 (2009).
Wyart, C. et al. Smelling a single component of male sweat alters levels of cortisol in women. J. Neurosci. 27, 1261–1265 (2007).
Preti, G., Wysocki, C. J., Barnhart, K. T. & Sondheimer, S. J. & Leyden, J.J. Male axillary extracts contain pheromones that affect pulsatile secretion of luteinizing hormone and mood in women recipients. Biol. Reprod. 68, 2107–2113 (2003).
Jacob, S., McClintock, M. K., Zelano, B. & Ober, C. Paternally inherited HLA alleles are associated with women’s choice of male odor. Nat. Genet. 30, 175–179 (2002).
Lundström, J. N. et al. Maternal status regulates cortical responses to the body odor of newborns. Front. Psychol. 4, 597 (2013).
Lemogne, C. et al. Congenital anosmia and emotion recognition: A case-control study. Neuropsychologia 72, 52–58 (2015).
Association, A. P. Diagnostic and Statistical Manual of Mental Disorders ( 5th edn. ) (American Psychiatric Association Publishing, Arlington, VA, USA, 2013).
Baron-Cohen, S. Social and pragmatic deficits in autism: cognitive or affective? J. Autism Dev. Disord. 18, 379–402 (1988).
Pause, B. M., Ohrt, A., Prehn, A. & Ferstl, R. Positive emotional priming of facial affect perception in females is diminished by chemosensory anxiety signals. Chem. Senses 29, 797–805 (2004).
Werling, D. M. & Geschwind, D. H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26, 146–153 (2013).
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J. & Clubley, E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord 31, 5–17 (2001).
Hus, V. & Lord, C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J. Autism Dev. Disord. 44, 1996–2012 (2014).
Grosser, B. I., Monti-Bloch, L., Jennings-White, C. & Berliner, D. L. Behavioral and electrophysiological effects of androstadienone, a human pheromone. Psychoneuroendocrinology 25, 289–299 (2000).
Frumin, I. & Sobel, N. in Pheromone Signaling 373–394 (Humana Press, Totowa, NJ, USA, 2013).
Bensafi, M. et al. Sex-steroid derived compounds induce sex-specific effects on autonomic nervous system function in humans. Behav. Neurosci. 117, 1125–1134 (2003).
Klein, B. et al. Activation of the mouse odorant receptor 37 subsystem coincides with a reduction of novel environment-induced activity within the paraventricular nucleus of the hypothalamus. Eur. J. Neurosci. 41, 793–801 (2015).
Hoppe, R., Lambert, T. D., Samollow, P. B., Breer, H. & Strotmann, J. Evolution of the “OR37” subfamily of olfactory receptors: a cross-species comparison. J. Mol. Evol. 62, 460–472 (2006).
de Lacy Costello, B. et al. A review of the volatiles from the healthy human body. J. Breath Res. 8, 014001 (2014).
Lang, P. J., Bradley, M. M. & Cuthbert, B. N. Emotion, attention, and the startle reflex. Psychol. Rev. 97, 377–395 (1990).
Bernier, R., Dawson, G., Panagiotides, H. & Webb, S. Individuals with autism spectrum disorder show normal responses to a fear potential startle paradigm. J. Autism Dev. Disord. 35, 575–583 (2005).
Prehn, A., Ohrt, A., Sojka, B., Ferstl, R. & Pause, B. M. Chemosensory anxiety signals augment the startle reflex in humans. Neurosci. Lett. 394, 127–130 (2006).
Senju, A. Atypical development of spontaneous social cognition in autism spectrum disorders. Brain Dev. 35, 96–101 (2013).
Li, W., Moallem, I., Paller, K. A. & Gottfried, J. A. Subliminal smells can guide social preferences. Psychol. Sci. 18, 1044–1049 (2007).
Cecchetto, C., Rumiati, R. I. & Parma, V. Relative contribution of odour intensity and valence to moral decisions. Perception 46, 447–474 (2017).
Parma, V., Bulgheroni, M., Tirindelli, R. & Castiello, U. Body odors promote automatic imitation in autism. Biol. Psychiatry 74, 220–226 (2013).
Woo, C. C., Donnelly, J. H., Steinberg-Epstein, R. & Leon, M. Environmental enrichment as a therapy for autism: a clinical trial replication and extension. Behav. Neurosci. 129, 412–422 (2015).
Pelphrey, K. A. et al. Visual scanning of faces in autism. J. Autism Dev. Disord 32, 249–261 (2002).
Philip, R. C. et al. Deficits in facial, body movement and vocal emotional processing in autism spectrum disorders. Psychol. Med. 40, 1919–1929 (2010).
Secundo, L., Snitz, K. & Sobel, N. The perceptual logic of smell. Curr. Opin. Neurobiol. 25, 107–115 (2014).
Ashwin, C. et al. Enhanced olfactory sensitivity in autism spectrum conditions. Mol. Autism 5, 53 (2014).
Rozenkrantz, L. et al. A mechanistic link between olfaction and autism spectrum disorder. Curr. Biol. 25, 1904–1910 (2015).
Halladay, A. K. et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 6, 36 (2015).
Savic, I., Berglund, H., Gulyas, B. & Roland, P. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron 31, 661–668 (2001).
Hazen, E. P., Stornelli, J. L., O’Rourke, J. A., Koesterer, K. & McDougle, C. J. Sensory symptoms in autism spectrum disorders. Harv. Rev. Psychiatry 22, 112–124 (2014).
Wyatt, T. D. The search for human pheromones: the lost decades and the necessity of returning to first principles. Proc. Biol. Sci. 282, 20142994 (2015).
Green, S. R., Kragel, P. A., Fecteau, M. E. & LaBar, K. S. Development and validation of an unsupervised scoring system (Autonomate) for skin conductance response analysis. Int. J. Psychophysiol. 91, 186–193 (2014).
Pause, B. M., Adolph, D., Prehn-Kristensen, A. & Ferstl, R. Startle response potentiation to chemosensory anxiety signals in socially anxious individuals. Int. J. Psychophysiol. 74, 88–92 (2009).
Blumenthal, T. D. et al. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42, 1–15 (2005).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Harris, H. et al. Perceptual learning in autism: over-specificity and possible remedies. Nat. Neurosci. 18, 1574–1576 (2015).
Lawson, R. P., Mathys, C. & Rees, G. Adults with autism overestimate the volatility of the sensory environment. Nat. Neurosci. 20, 1293–1299 (2017).
Simonsohn, U., Nelson, L. D. & Simmons, J. P. P-curve and effect size: Correcting for publication bias using only significant results. Perspect. Psychol. Sci. 9, 666–681 (2014).
Bensafi, M., Brown, W. M., Khan, R., Levenson, B. & Sobel, N. Sniffing human sex-steroid derived compounds modulates mood, memory and autonomic nervous system function in specific behavioral contexts. Behav. Brain Res. 152, 11–22 (2004).
Levenson, R. W., Ekman, P. & Friesen, W. V. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology 27, 363–384 (1990).
Acknowledgements
We thank H. Breer and J. Strotmann for suggesting that we investigate hexadecanal in humans. We thank Ziv and all the instructors and management at Paradive for their gracious hospitality and help. This work was supported by ISF grant #1379/15, ERC Advanced grant #670798 SocioSmell, grant #712254 from the US Air Force Office of Scientific Research Program on Trust and Influence and by the McEwen Fund.
Author information
Authors and Affiliations
Contributions
Developed the idea: Y.E.-S. and N.S. Ran experiments: Y.E.-S., D.A., A.E., V.B., L.R., E.M., L.P., T.S. and N.S. Developed devices: O.P., D.H., and N.S. Analyzed data: Y.E.-S., N.S., A.R. and O.P. Wrote the paper: Y.E.-S., N.S. and O.P.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated Supplementary Information
Supplementary Figure 1 Illustration of the shirt-sniffing device (SSD)
To standardize body-odor sampling we developed the SSD. This consisted of a glass jar containing the T-shirt, with an air intake port via soda lime filter, and air sampling port via one-way flap valve into individual-use airtight nose mask. This arrangement assured that environmental odors didn't contaminate the sample during the sampling process. The recognizable person in the figure is a co-author and not a participant.
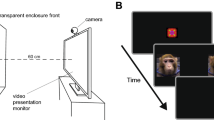
Supplementary Figure 2 Experimental time-course for Stroop and Faces experiments with fear sweat
Each participant tested twice on the same day, once with the fear sweat and once with control, counterbalanced for order, participants blind to condition. Fearful face image from the NimStim set of facial expressions (1). All other photos and graphics: Ofer Perl. 1. Tottenham, N., Tanaka, J., Leon, A.C., McCarry, T., Nurse, M., Hare, T.A., Marcus, D.J., Westerlund, A., Casey, B.J., Nelson, C.A. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3):242-9.
Supplementary Figure 3 ER-EDA effects not driven by sniffing
The extent of change in ER-EDA as a function of the extent of change in sniff airflow in ASD. There was a slight trend for increased sniff modulation to the smell of fear in ASD vs. TD. Thus, one could raise the possibility that this is driving the ER-EDA effects. To address this possibility, we tested for a relation between change in ER-EDA and change in nasal airflow in ASD, yet observed no relation (r = 0.033, p = 0.9).
Supplementary Figure 4 ER-EDA during Stroop
The Stroop task contained only one trial, and was thus not optimized for this analysis. Nevertheless, we observe a trend very similar to that seen in the Faces task, whereby the smell of fear increased arousal in TD but decreased it in ASD. An ANOVA applied to the EDA trace obtained over the ~40 sec task revealed no main effect (all F1,33 < 0.3, all p > 0.6), yet near significant interaction (F1,33 = 4.0, p = 0.054). This reflected that exposure to the smell of fear relative to control non- significantly increased EDA in TD (normalized Trough to Peak, Control = 0.94 ± 0.38 Nμs, Fear = 1.17 ± 0.40 Nμs, t(19) =1.6 p = 0.12) yet exposure to the smell of fear relative to control non- significantly decreased EDA in ASD (normalized Trough to peak, Control = 1.15 ± 0.48 Nμs, Fear = 0.89 ± 0.47 Nμs, t(14) = 1.3, p = 0.24). All tests were two-tailed, all centers reflect mean, all error bars reflect SEM.
Supplementary Figure 5 Relation between ER-EDA and AQ
The relation between change in ER-EDA in the Faces task and AQ scores of all participants, TD (N =19) and ASD (n =17): r = −0.33, p = 0.043. This effect does not survive correction for the multiple comparisons involved.
Supplementary Figure 6 Manikin experimental set-up. A
The actual location of the manikins in the experimental room. Note that a thin wall separates the two manikins, and each has a highflow exhaust vent directly overhead. This design prevented contamination. Also note that each manikin is identified by nametag, which is switched across manikins across experiments. B. The typical posture of a participant interacting with the manikins. Because the manikin voice volume was very low, participants tended to put their ears up close to the manikin, thus putting their nose in line with the emitted chemosignal.
Supplementary Figure 7 Ratings of AND intensity pleasantness and familiarity
Mean ± SEM Ratings of stimulus intensity pleasantness and familiarity. Reported perception was similar for the clove carrier alone and for the clove carrier with AND in both TD (n = 23) and ASD (n = 17) groups. A two-way repeated-measures ANOVA on the ratings of stimulus intensity pleasantness and familiarity applied by the participants directly before the ensuing task, with factors of Condition (AND/control), Descriptor (Intensity/Familiarity/Pleasantness) and a categorical independent factor of Group (TD/ASD) revealed no main effect (all F1,38 < 2.8, all p > 0.1), and no interaction (all F2,76 < 1.4, all p > 0.26). In other words, verbally reported perception was similar for the clove carrier alone and for the clove carrier with AND in both TD and ASD. All tests were two-tailed, all centers reflect mean, all error bars reflect SEM.
Supplementary Figure 8 Experimental timeline with Androstadienone
Each participant tested on separate days at the same time, once with the chemosignal (AND) and once with control, counterbalanced for order, double-blind. All photos and graphics: Ofer Perl.
Supplementary Figure 9 AND increased arousal in TD but not in ASD. A
The influence of AND on ongoing changes in physiological index. Each circle (TD = clear, ASD = red) represents the change in physiological index of a single participant following AND (Y-axis) and Control (X-axis). The diagonal line reflects the unit slope line (x=y) (n = 23 TD, 17 ASD). B. Change in physiological index (summated results of the data in A). The composite physiological index combined the different physiological parameters (NS-EDA, Change in temperature and HRV) with equal weighting (1). An ANOVA with factors of Condition (AND/Control), Time (Before/After CP) and Group (ASD/TD) revealed a significant main effect of Time (F1,38 = 57.8, p < 10−8, Before CP = −0.66 ± 1.3 AU, After CP = 0.66 ± 1.2 AU) and a significant interaction of Group X Condition X Time (F1,38 = 7.8, p = 0.008, non-parametric reanalysis: U = 99, Z = 2.6, p = 0.008, Cohen's d' = 0.92). This reflected a similar physiological index change across the experiment in TD and ASD in response to the control odor (TD = 0.97 ± 1.39 AU, ASD = 1.31 ± 0.95 AU, t(38) = 0.85, p = 0.40). Yet, the addition of undetected AND significantly reversed the drop in TD (Δ control = 0.97 ± 1.39 AU, Δ AND = 2.10 ± 1.69 AU, t(22) = 2.5, p = 0.02), but had no influence in ASD (Δ control = 1.31 ± 0.95 AU, Δ AND = 0.71 ± 1.46 AU, t(16) = 1.6, p = 0.14). All tests were two-tailed, all centers reflect mean, all error bars reflect SEM. ** = p < 0.01. 1. Bensafi, M., Brown, W. M., Khan, R., Levenson, B. & Sobel, N. Sniffing human sex-steroid derived compounds modulates mood, memory and autonomic nervous system function in specific behavioral contexts. Behav Brain Res 152, 11–22 (2004).
Supplementary Figure 10 Influence of AND on Testosterone
A. The influence of AND on testosterone of TD (n = 23, clear) and ASD (n =15, red). (a) Each circle represents the testosterone ratio of a single participant following AND (Y-axis) and Control (X-axis). B. The delta testosterone. Measurements of testosterone in saliva revealed a pattern similar to that evidenced in physiological monitoring. An ANOVA with factors of Condition (AND/Control), Time (Start/End of the experiment) and Group (ASD/TD) revealed a main effect of Time (F1,36 = 10.1, p = 0.003, Testosterone concentration pg/mL Start = 168.7 ± 72.7, End = 218.6 ± 87.5) and a significant interaction of Condition X Time X Group (F1,36 = 4.8, p = 0.035, Cohen's d' = 0.72). This reflected that in both TD and ASD testosterone similarly increased across the experiment. The addition of undetected AND slightly further increased testosterone in TD (Testosterone concentration, Δ Control = 35.0 ± 72.6 pg/mL, ΔAND = 87.9 ± 130.6 pg/mL, t(22) = 1.5, p = 0.14), but slightly decreased testosterone in ASD (Testosterone concentration, Δ Control = 73.5 ± 147.6 pg/mL, Δ AND = −8.7 ± 165.7 pg/mL, t(14) = 1.5, p = 0.15). This is consistent with the EDA results reported in the main text whereby AND increased arousal in TD and decreased it in ASD. All tests were two-tailed, all centers reflect mean, all error bars reflect SEM. * = p < 0.05.
Supplementary Figure 11 Ratings of HEX intensity pleasantness and familiarity
Mean ± SEM Ratings of stimulus intensity pleasantness and familiarity. Verbally reported perception was similar for the clove carrier alone and for the clove carrier with HEX in both TD (n = 16) and ASD (n = 17) groups. A two-way repeated-measures ANOVA on the ratings of stimulus intensity pleasantness and familiarity applied by the participants during the ensuing tasks with factors of Condition (HEX/control), Descriptor (intensity/familiarity/pleasantness) and a categorical independent factor of Group (TD/ASD). This revealed no main effects (all F < 2.6, all p > 0.1), no interaction of Descriptor X Group X Condition (F2,62 = 0.9, p = 0.41), and a trend towards an interaction of Group X Condition (F1,31 = 3.5, p = 0.072). In other words, verbally reported perception was similar for the clove carrier alone and for the clove carrier with HEX in both TD and high-function ASD. All tests were two-tailed, all centers reflect mean, all error bars reflect SEM.
Supplementary Figure 12 Experimental timeline with Hexadecanal
Each participant tested on separate days at the same time, once with the chemosignal (HEX) and once with control, counterbalanced for order, participants blind to condition. All photos and graphics: Ofer Perl.
Supplementary Figure 13 Hex increased sniff duration in ASD but not in TD
A. The influence of HEX on the sniff duration (ms) relative to control of TD (n = 15, white) and ASD (n = 12, red). Each circle represents mean sniff duration (ms) of a single participant following HEX (Y-axis) and Control (X- axis) B. Mean ± SEM Sniff duration. Because both ASD women (and two of the ASD men) refused to wear the nasal cannula, we compared the ASD group to the TD men only. A one way repeated-measures ANOVA applied to each of the standard sniff parameters (Volume/Duration/Peak), with conditions of Condition (HEX/Control) and categorical independent factor of Group (TD/ASD) revealed no main effects or interactions for Volume and Peak (all F1,25 < 2.3, p > 0.14),but for Duration we observed a significant main effect of Group (F1,25 = 7.4, p = 0.011), and a significant Condition X Group interaction (F1,25 = 4.9, p = 0.036, Cohen's d' = 0.87). This reflected that in ASD but not in TD mean sniff duration was longer for undetected HEX than for control (ASD: Control = 1396.5 ± 454.1 ms, HEX = 1701.7 ± 481.3 ms, t(11) = 2.9, p = 0.014, TD: Control = 2212.1 ± 617.1 ms, HEX = 2165.5 ± 864.8 ms, t(14) = 0.41, p = 0.69). This result further implies that the results of this study are not a reflection of an inability of the ASD brain to register the stimulus. The ASD brain differed between HEX and control as evidenced here in the longer duration sniffs, yet the autonomic impact in ASD was opposite that of TD. All tests were two-tailed, all centers reflect mean, all error bars reflect SEM. * = p < 0.05.
Supplementary Figure 14 NS-EDA decreased following HEX in TD but not in ASD
Mean ± SEM NS-EDA. Mean NS-EDA decreased following exposure to HEX (red) than for control (blue) in TD (n =17) but not in ASD (n = 15). An ANOVA applied to the standard deviation in skin conductance (NS-EDA) revealed a significant main effect of Time (F1,30= 14.4, p < 10−4) and a significant interaction of Condition X Time X Group (F1,30= 4.7, p = 0.038, Cohen's d' = 0.75) showing that HEX reduced NS-EDA in TD (Δ Control = 0.14 ± 0.21 μs, Δ HEX = −0.03 ± 0.13 μs, t(16) = 2.8, p = 0.012) but had no influence on ASD (Δ Control = 0.13 ± 0.16 μs, Δ HEX = 0.21 ± 0.38 μs, t(14)=0.78, p = 0.45). This ongoing measure is consistent with the startle response to imply an autonomic calming effect of HEX in TD but not ASD. All tests were two-tailed, all centers reflect mean, all error bars reflect SEM. * = p < 0.05.
Supplementary Figure 15 Startle effects not driven by sniffing
The extent of change in EMG as a function of the extent of change in sniff duration in ASD (n = 12). Given the altered sniffing for HEX one could raise the possibility that this is driving the startle response. To address this possibility, we tested for a relation between change in startle and change in nasal airflow in ASD, yet observed no relation (r = 0.063, p = 0.85).
Supplementary Figure 16 The distribution of p values following reiteratively removing 2 TD men and 2 ASD men from the startle analysis
The red line represents the p value following removing the women (2 TD and 2 ASD) from the startle analysis. To ask whether the effects of HEX on startle were gender-specific we excluded the women from analysis of that one study (2 TD, 2 ASD). The ANOVA revealed a near significant interaction of Condition X Group (F1,26 = 4.1, p = 0.054). Notably, this analysis resulted in decreased significance compared with the original analysis that included all participants. In turn, this has now reduced our sample size by 4 participants. To estimate whether this was the effect of gender or the effect of sample size, we reiteratively removed 4 men (2 TD and 2 ASD) 1000 times, and tested the distribution of p values. We obtained a mean of p = 0.016 ± 0.012, which is indeed significantly different from 0.054. Thus, we conclude that the effect was in the same direction in men and women, but stronger in women that participated in the experiment.
Supplementary Figure 17 HEX impacted self-reported mood in ASD but not TD
A. The influence of HEX on the positive mood rating relative to control of TD (n =16, white) and ASD (n =17, red). Each circle represents the positive mood rating of a participant following HEX (y-axis) and Control (x-axis). B. The positive mood of the ASD participants increased significantly compared with TD (F1,31 = 7.8, p = 0.009). A two-way repeated-measures ANOVA with conditions of Condition (Hexadecanal/Control), Time (Beginning/End of the experiment) and a categorical independent factor of Group (TD/ASD) was applied to each of the mood subscales (Positive/Negative low/Negative high/Sexual arousal). The ANOVA on Positive mood revealed a significant interaction of Condition X Time X Group (F 1,31 = 7.8, p = 0.009, Cohen's d' = 0.97) (Supplementary Figure 19). This reflected a significant increase in the positive mood of the ASD group alone following exposure to undetected HEX (ASD: Δ Hex = 86.3 ± 174.1, Δ Control = −68.5 ± 128.1, t(16) = 4.1, p = 0.0008, TD: Δ Hex = −37.1 ± 136.9, Δ Control = 2.0 ± 174.4, t(15) = 0.65, p = 0.52). ANOVAs on the other subscales revealed no interaction or main effect that involved Condition (all F < 2.5, all p > 0.1). In other words, the influence of HEX on self-reported mood in this study was limited to one subscale alone. Although this again points to dissociable impact of HEX in TD and ASD, we are cautious in interpretation of this result. This is because unlike the psychophysiological results that emerged again and again throughout all experiments in this study, this self-reported mood result is the only significant case throughout the study. Although its extent is such that it in fact survives correction for multiple comparisons following from the above, we nevertheless retain caution in interpretation. All tests were two-tailed, all centers reflect mean, all error bars reflect SEM. ** = p < 0.01.
Supplementary Figure 18 The distribution of the reported p-values throughout the manuscript
We take a meta-analysis approach to the experiments we conducted. In order to verify that our reported p values in the manuscript are not selective and to test whether our findings across experiments reflect true effects, we applied p-curve analysis (1). We found that the p-curve of all our experiments combined is significantly right skewed (Full p-curve, Z = −7.24, p < 10−5, Half p-curve, Z = −5.88, p < 10−5), indicating overall evidential value of the reported results. 1. Simonsohn, U., Nelson, L. D. & Simmons, J. P. P-curve and effect size: Correcting for publication bias using only significant results. Perspectives on Psychological Science 9, 666–681 (2014).
Supplementary Figure 19 Mouse trajectory presentation
The following heat maps depict an accumulation of all mouse locations on the task screen within 1000 ms from trial onset. Each of the 12 sub-plots corresponds to mouse movement of a specific experimental group. Color code denotes the number of accumulated counts of a certain pixel coordinate being “visited” by the mouse cursor throughout all trials. One can observe fine single-trial trajectories traveling from the center to arrive at the expected location of the target. From top to bottom: trials of TD participants approaching the Smell of Fear manikin, trials of TD participants approaching the control manikin, trials of ASD participants approaching the Smell of Fear manikin, trials of ASD participants approaching the control manikin. Horizontal division into sub plots is based on the hint given by the manikin. From left to right: Hint to left target, hint to right target and a superposition of both hints directions created by adding of left target and a mirror image of right target to result in a “go towards target” disregarding screen orientation. Lastly, in the rightmost panel is the comparison of response to Smell of fear vs. control from both the TD group (top) and ASD group (bottom). Color code denotes the delta of accumulated trajectories per group such that “hot” colors denote a higher number of trajectories that went through the pixel coordinate while “cold” colors denote less “visits” of a certain pixel coordinate.
Supplementary Figure 20 Manikin voice selection
The results of an online study comparing the trustworthiness associated with 6 different potential voices for the manikins. All centers reflect mean, all error bars reflect SEM (n = 198).
Supplementary information
Supplementary information
Supplementary Figures 1–20 and Supplementary Table 1
All Raw Data
Supplementary Dataset
Supplementary Software
Supplementary Software
Rights and permissions
About this article
Cite this article
Endevelt-Shapira, Y., Perl, O., Ravia, A. et al. Altered responses to social chemosignals in autism spectrum disorder. Nat Neurosci 21, 111–119 (2018). https://doi.org/10.1038/s41593-017-0024-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-017-0024-x
This article is cited by
-
Sensory Gating in Neurodevelopmental Disorders: A Scoping Review
Research on Child and Adolescent Psychopathology (2023)
-
Assessing olfactory, memory, social and circadian phenotypes associated with schizophrenia in a genetic model based on Rim
Translational Psychiatry (2021)
-
The potential for retronasally delivered olfactory stimuli to assess psychiatric conditions
Current Psychology (2021)
-
Genetic influences of autism candidate genes on circuit wiring and olfactory decoding
Cell and Tissue Research (2021)
-
Computational identification of variables in neonatal vocalizations predictive for postpubertal social behaviors in a mouse model of 16p11.2 deletion
Molecular Psychiatry (2021)