Abstract
All animals must detect noxious stimuli to initiate protective behavior, but the evolutionary origin of nociceptive systems is not well understood. Here we show that noxious heat and irritant chemicals elicit robust escape behaviors in the planarian Schmidtea mediterranea and that the conserved ion channel TRPA1 is required for these responses. TRPA1-mutant Drosophila flies are also defective in noxious-heat responses. We find that either planarian or human TRPA1 can restore noxious-heat avoidance to TRPA1-mutant Drosophila, although neither is directly activated by heat. Instead, our data suggest that TRPA1 activation is mediated by H2O2 and reactive oxygen species, early markers of tissue damage rapidly produced as a result of heat exposure. Together, our data reveal a core function for TRPA1 in noxious heat transduction, demonstrate its conservation from planarians to humans, and imply that animal nociceptive systems may share a common ancestry, tracing back to a progenitor that lived more than 500 million years ago.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kremeyer, B. et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66, 671–680 (2010).
Viswanath, V. et al. Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823 (2003).
Jordt, S. E. et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004).
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004).
Macpherson, L. J. et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 15, 929–934 (2005).
Bautista, D. M. et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 102, 12248–12252 (2005).
Bautista, D. M. et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 (2006).
Macpherson, L. J. et al. An ion channel essential for sensing chemical damage. J. Neurosci. 27, 11412–11415 (2007).
Neely, G. G. et al. TrpA1 regulates thermal nociception in Drosophila. PLoS One 6, e24343 (2011).
Zhong, L. et al. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP channel. Cell Reports 1, 43–55 (2012).
Kang, K. et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464, 597–600 (2010).
Chatzigeorgiou, M. et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 13, 861–868 (2010).
Laursen, W. J., Bagriantsev, S. N. & Gracheva, E. O. TRPA1 channels: chemical and temperature sensitivity. Curr. Top. Membr. 74, 89–112 (2014).
Chen, J. et al. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 4, 2501 (2013).
Saito, S. et al. Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Mol. Biol. Evol. 31, 708–722 (2014).
Gracheva, E. O. et al. Molecular basis of infrared detection by snakes. Nature 464, 1006–1011 (2010).
Saito, S. et al. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J. Biol. Chem. 287, 30743–30754 (2012).
Oda, M., Kurogi, M., Kubo, Y. & Saitoh, O. Sensitivities of two zebrafish TRPA1 paralogs to chemical and thermal stimuli analyzed in heterologous expression systems. Chem. Senses 41, 261–272 (2016).
Prober, D. A. et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J. Neurosci. 28, 10102–10110 (2008).
Kohno, K., Sokabe, T., Tominaga, M. & Kadowaki, T. Honey bee thermal/chemical sensor, AmHsTRPA, reveals neofunctionalization and loss of transient receptor potential channel genes. J. Neurosci. 30, 12219–12229 (2010).
Wang, G. et al. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur. J. Neurosci. 30, 967–974 (2009).
Kang, K. et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 481, 76–80 (2011).
Dunn, C. W., Giribet, G., Edgecombe, G. D. & Hejnol, A. Animal phylogeny and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 45, 371–395 (2014).
Newmark, P. A., Reddien, P. W., Cebrià, F. & Sánchez Alvarado, A. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc. Natl. Acad. Sci. USA 100 (Suppl. 1), 11861–11865 (2003).
Wenemoser, D., Lapan, S. W., Wilkinson, A. W., Bell, G. W. & Reddien, P. W. A molecular wound response program associated with regeneration initiation in planarians. Genes Dev. 26, 988–1002 (2012).
Gallio, M., Ofstad, T. A., Macpherson, L. J., Wang, J. W. & Zuker, C. S. The coding of temperature in the Drosophila brain. Cell 144, 614–624 (2011).
Inoue, T., Yamashita, T. & Agata, K. Thermosensory signaling by TRPM is processed by brain serotonergic neurons to produce planarian thermotaxis. J. Neurosci. 34, 15701–15714 (2014).
Wang, H., Schupp, M., Zurborg, S. & Heppenstall, P. A. Residues in the pore region of Drosophila transient receptor potential A1 dictate sensitivity to thermal stimuli. J. Physiol. (Lond.) 591, 185–201 (2013).
Cordero-Morales, J. F., Gracheva, E. O. & Julius, D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc. Natl. Acad. Sci. USA 108, E1184–E1191 (2011).
Kwon, Y., Shim, H.-S., Wang, X. & Montell, C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 11, 871–873 (2008).
Lee, Y. & Montell, C. Drosophila TRPA1 functions in temperature control of circadian rhythm in pacemaker neurons. J. Neurosci. 33, 6716–6725 (2013).
Ni, L. et al. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500, 580–584 (2013).
Niethammer, P., Grabher, C., Look, A. T. & Mitchison, T. J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 (2009).
Moreira, S., Stramer, B., Evans, I., Wood, W. & Martin, P. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol. 20, 464–470 (2010).
Pirotte, N. et al. Reactive oxygen species in planarian regeneration: an upstream necessity for correct patterning and brain formation. Oxid. Med. Cell. Longev. 2015, 392476 (2015).
Xu, C., Luo, J., He, L., Montell, C. & Perrimon, N. Oxidative stress induces stem cell proliferation via TRPA1/RyR-mediated Ca(2+) signaling in the Drosophila midgut. eLife 6, e22441 (2017).
Andersson, D. A., Gentry, C., Moss, S. & Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 28, 2485–2494 (2008).
Du, E. J. et al. Nucleophile sensitivity of Drosophila TRPA1 underlies light-induced feeding deterrence. eLife 5, e18425 (2016).
Guntur, A. R. et al. H2O2-sensitive isoforms of Drosophila melanogaster TRPA1 act in bitter-sensing gustatory neurons to promote avoidance of UV during egg-laying. Genetics 205, 749–759 (2017).
Guntur, A. R. et al. Drosophila TRPA1 isoforms detect UV light via photochemical production of H2O2. Proc. Natl. Acad. Sci. USA 112, E5753–E5761 (2015).
Kim, M. J. & Johnson, W. A. ROS-mediated activation of Drosophila larval nociceptor neurons by UVC irradiation. BMC Neurosci. 15, 14 (2014).
Birkholz, T. R. & Beane, W. S. The planarian TRPA1 homolog mediates extraocular behavioral responses to near-ultraviolet light. J. Exp. Biol. 220, 2616–2625 (2017).
Miyake, T. et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat. Commun. 7, 12840 (2016).
Kalyanaraman, B. et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 52, 1–6 (2012).
Albrecht, S. C., Barata, A. G., Grosshans, J., Teleman, A. A. & Dick, T. P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829 (2011).
Rzezniczak, T. Z., Douglas, L. A., Watterson, J. H. & Merritt, T. J. Paraquat administration in Drosophila for use in metabolic studies of oxidative stress. Anal. Biochem. 419, 345–347 (2011).
Kwan, K. Y. et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289 (2006).
Cochet-Escartin, O., Mickolajczyk, K. J. & Collins, E. M. Scrunching: a novel escape gait in planarians. Phys. Biol. 12, 056010 (2015).
Inoue, T., Hoshino, H., Yamashita, T., Shimoyama, S. & Agata, K. Planarian shows decision-making behavior in response to multiple stimuli by integrative brain function. Zoological letters 1, 7 (2015).
Umesono, Y., Tasaki, J., Nishimura, K., Inoue, T. & Agata, K. Regeneration in an evolutionarily primitive brain-the planarian Dugesia japonica model. Eur. J. Neurosci. 34, 863–869 (2011).
Robb, S. M. C., Gotting, K., Ross, E. & Sánchez Alvarado, A. SmedGD 2.0: the Schmidtea mediterranea genome database. Genesis 53, 535–546 (2015).
Acknowledgements
We thank D. Tracey and R. Carthew for reagents; A. Kuang and L. Vinson for technical assistance; L. Macpherson, D. Yarmolinsky, and members of the Gallio Lab for comments on the manuscript; I. Raman for technical advice, and M. Stensmyr for the kind gift of the fly drawing in Fig. 1. Work in the Gallio lab is supported by NIH grant R01NS086859 (to M.G.), the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust (to E.E.Z.), and by training grant 2T32MH067564 (to O.M.A.). Work in the Petersen Lab is supported by an NIH Director’s New Innovator award (1DP2DE024365-01).
Author information
Authors and Affiliations
Contributions
M.G. designed the study, analyzed the data, and wrote the paper with critical input from all authors; M.G. and O.M.A. designed and built the behavioral assays. O.M.A. performed all planarian behavioral experiments and electrophysiology and analyzed the corresponding data. E.E.Z. performed all fly rescue experiments and ROS assays and analyzed the corresponding data. A.P. cloned Smed-TRPA1, produced rescue constructs and transgenics, and analyzed sequences with help from C.P.P; A.P., O.M.A., and C.V.D. performed q-PCR and ISH experiments. E.E.Z. and O.M.A. generated human-TRPA1-expressing flies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information.
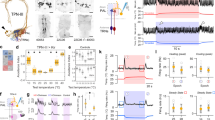
Supplementary Figure 1 Design of the arena used to measure heat avoidance behavior in planarians (the Planariometer).
a) Planariometer design. In each trial two opposing tiles are set to 24°C and two to 32°C (noxious heat). Animal movement is then recorded for 4 minutes. The spatial configuration of hot and cool tiles is then reversed for 4 additional minutes (and a second movie acquired) to control for potential spatial biases. The hot and cold tiles are covered by thin anodized aluminum foil; on it, a hydrophobic ink pen is used to create a circular barrier. A thin film of water (1-2 mm) forms a central pool in which the worms can move freely. The experiment is conducted in the dark, under IR-LED illumination; movies are acquired using an IR-sensitive CCD camera. b) Images acquired using a thermal imaging camera, showing sharp thermal boundaries between hot and cool quadrants in the two spatial configurations used for each experiment. c) Snapshots from video recordings of planarian movement, corresponding to the thermal configurations shown in b.
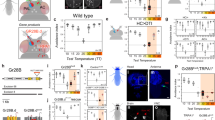
Supplementary Figure 2 Smed-TRPA1 RNAi does not impair negative phototaxis.
a) A simple assay to measure negative phototaxis in planarians. Worms are released in chamber 1 either in the dark or in bright room light, while chamber 2 is kept in constant darkness. b) Both controls and experimental animals avoid the light, and efficiently escape into the dark chamber (chamber 2). Outer boxes = +- STD; Inner boxes = 95% Confidence Interval.
Supplementary Figure 3 Dye, imaging, and feeding controls.
a) The fluorogenic ROS dye, carboxy-H2DCFDA does not increase in fluorescence as a result of heating. Drosophila tissue (larval salivary glands) that was heat-killed prior to dye loading did not display the prominent heat-dependent increase in fluorescent observed in live tissue (see Figure 6g). b) Carboxy-H2DCFDA fluorescence increases are not due to imaging conditions. Imaging dye-loaded, live tissue (larval salivary glands) at constant temperature, produces a modest fluorescent changes (compare to Figure 6g). In all panels: thick line=average, grey shading= +-STD, imaging conditions are as in Figure 6g. c) Feeding controls, related to the experiments in Figure 6i. A green food dye demonstrates that, prior to their performance in the arena, flies indeed ingest H2O2 or Paraquat laced food.
Supplementary Figure 4 The genetically encoded ratiometric H2O2 indicator roGFP2-Orp1 reports increase in H2O2 levels in fly larvae upon brief (~5 s) exposure to noxious heat.
A, B and C above are schematics of the experiment and correspond to the x-axis legend of the boxplot. A is the negative control (no treatment), while C is the positive control (H2O2 treatment). Fly genotype: tub-cyto-roGFP2-Orp1, (a transgenic line expressing the H2O2 indicator roGFP2-Orp1 in all cells, under a tubulin promoter). Red line = mean; Outer boxes = +- STD; Inner boxes = 95% Confidence Interval; from left: *P=0.006 and *P=1.12 x e-4 in unpaired t-tests; t(15)=-3.2 n=9,8; t(13)=-5.44 n=9,6. Control fluorescence (i.e. of untreated tissue) was set to 1.
Supplementary Figure 5 Knockdown of Smed-TRPA1 reduces scrunching following a physical injury (i.e., a tail snip).
a) Selected frames from a video including and immediately following a tail snip (shown at 1, time=0). “Scrunching” is described as the unusual gate planarians engage in following injury, and is characterized by strong contraction/expansion cycles – quite different from the planarian’s normal smooth gliding movement. b) Normalized body area throughout representative movies for control (blue, UNC22 RNAi) and Smed-TRPA1 RNAi (red; for each animal 1=average length across the movie, movie=15 seconds at 3 frames/sec). c) Amplitude of contractions calculated throughout the movie. Line=mean. Outer boxes = +- STD; Inner boxes = 95% Confidence Interval; *P=0.0022 t(13)=3.79, unpaired t-test; n=7,8
Supplementary information.
Supplementary Text and Figures
Supplementary Figures 1–5
Supplementary Video 1 A behavioural assay designed to measure noxious heat avoidance in Planarian worms.
The videos show heat avoidance behaviour of control worms as well as Smed-TRPA1 RNAi worms (S. mediterranea; videos are accelerated 24X). In this arena, worms are given a choice between 24°C (top right and bottom left tiles) and 32°C (top left and bottom right tiles). Control worms are essentially confined to the cool quadrants; in contrast Smed-TRPA1 RNAi planarians glide around the chamber but do not appear confined to the 24°C regions (i.e. they are defective in heat avoidance)
Supplementary Video 2 A behavioural assay designed to measure AITC avoidance in Planarian worms.
This set up consists of shallow circular chambers filled with water and interconnected by narrow corridors that worms do not readily traverse (note that connected chambers form an inverted U shape, i.e. chambers 1 at the bottom right and 4 at the bottom left do not communicate). At the beginning of each experiment, worms are introduced in chamber 1 in the presence of either a mock Agar pellet or of a pellet laced with AITC. AITC avoidance is apparent as the worms perform quick withdrawal manoeuvres in the vicinity of the AITC pellet and eventually escape to distant chambers (videos are accelerated 24X; Note that the setup used to acquire the data presented in Figure 2 was a simplified version, with two chambers rather than four)
Rights and permissions
About this article
Cite this article
Arenas, O.M., Zaharieva, E.E., Para, A. et al. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat Neurosci 20, 1686–1693 (2017). https://doi.org/10.1038/s41593-017-0005-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-017-0005-0
This article is cited by
-
Sonogenetic control of mammalian cells using exogenous Transient Receptor Potential A1 channels
Nature Communications (2022)
-
Robustness and plasticity in Drosophila heat avoidance
Nature Communications (2021)
-
Independent evolution of pain insensitivity in African mole-rats: origins and mechanisms
Journal of Comparative Physiology A (2020)
-
Pulse magnetization elicits differential gene expression in the central nervous system of the Caribbean spiny lobster, Panulirus argus
Journal of Comparative Physiology A (2020)
-
Calcium ions in the aquatic environment drive planarians to food
Zoological Letters (2019)