Abstract
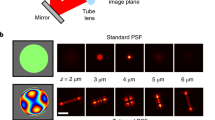
Single-molecule localization microscopy in a typical wide-field setup has been widely used for investigating subcellular structures with super resolution; however, field-dependent aberrations restrict the field of view (FOV) to only tens of micrometers. Here, we present a deep-learning method for precise localization of spatially variant point emitters (FD-DeepLoc) over a large FOV covering the full chip of a modern sCMOS camera. Using a graphic processing unit-based vectorial point spread function (PSF) fitter, we can fast and accurately model the spatially variant PSF of a high numerical aperture objective in the entire FOV. Combined with deformable mirror-based optimal PSF engineering, we demonstrate high-accuracy three-dimensional single-molecule localization microscopy over a volume of ~180 × 180 × 5 μm3, allowing us to image mitochondria and nuclear pore complexes in entire cells in a single imaging cycle without hardware scanning; a 100-fold increase in throughput compared to the state of the art.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are publicly available at Zenodo. For Fig. 3, raw data, aberration maps, ground-truth list and example network models of the dataset with normal aberration and medium SNR are available at https://doi.org/10.5281/zenodo.7310921; For Fig. 4, raw data of two cropped ROIs, aberration maps and example network model are available at https://doi.org/10.5281/zenodo.7299483; the raw bead stacks are available at https://doi.org/10.5281/zenodo.7302064. For Fig. 6, the first 10,000 frames of the raw data, aberration maps and example network model are available at https://doi.org/10.5281/zenodo.7299064; the raw bead stacks are available at https://doi.org/10.5281/zenodo.7300138. For Supplementary Fig. 5, the raw data, uniform aberration maps and example network model are available at https://doi.org/10.5281/zenodo.7299197. The statistical source data for Figs. 1–4 and Extended Data Figs. 1, 5, 8 and 9 are attached. Other datasets are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Source code of FD-DeepLoc is provided in Supplementary Code 1 and updated versions are available at: https://github.com/Li-Lab-SUSTech/FD-DeepLoc. The microscope acquisition software was developed with Micro-manager v.2.0 (http://micro-manager.org) and EMU v.1.1 (https://github.com/jdeschamps/htSMLM). The custom localization data analysis and rendering software is available at https://github.com/jries/SMAP.
References
Chi, W. et al. Descriptor Δ G C‐O Enables the quantitative design of spontaneously blinking rhodamines for live‐cell super‐resolution imaging. Angew. Chem. Int. Ed. 59, 20215–20223 (2020).
Pegoraro, G. & Misteli, T. High-throughput imaging for the discovery of cellular mechanisms of disease. Trends Genet. 33, 604–615 (2017).
Holden, S. J. et al. High-throughput 3D super-resolution microscopy reveals Caulobacter crescentus in vivo Z-ring organization. Proc. Natl Acad. Sci. USA 111, 4566–4571 (2014).
Mahecic, D. et al. Homogeneous multifocal excitation for high-throughput super-resolution imaging. Nat. Methods 17, 726–733 (2020).
Beghin, A. et al. Localization-based super-resolution imaging meets high-content screening. Nat. Methods 14, 1184–1190 (2017).
Barentine, A. E. S. et al. An integrated platform for high-throughput nanoscopy. Preprint at bioRxiv https://doi.org/10.1101/606954 (2022).
Du, Y. et al. Computational framework for generating large panoramic super-resolution images from localization microscopy. Biomed. Opt. Express 12, 4759 (2021).
Zhao, Z., Xin, B., Li, L. & Huang, Z. High-power homogeneous illumination for super-resolution localization microscopy with large field-of-view. Opt. Express 25, 13382 (2017).
Diekmann, R. et al. Chip-based wide field-of-view nanoscopy. Nat. Photonics 11, 322–328 (2017).
Douglass, K. M., Sieben, C., Archetti, A., Lambert, A. & Manley, S. Super-resolution imaging of multiple cells by optimized flat-field epi-illumination. Nat. Photonics 10, 705–708 (2016).
Mau, A., Friedl, K., Leterrier, C., Bourg, N. & Lévêque-Fort, S. Fast widefield scan provides tunable and uniform illumination optimizing super-resolution microscopy on large fields. Nat. Commun. 12, 3077 (2021).
Lohmann, A. W. & Paris, D. P. Space-variant image formation. J. Opt. Soc. Am. 55, 1007 (1965).
Diezmann, A., von, Lee, M. Y., Lew, M. D. & Moerner, W. E. Correcting field-dependent aberrations with nanoscale accuracy in three-dimensional single-molecule localization microscopy. Optica 2, 985 (2015).
Yan, T., Richardson, C. J., Zhang, M. & Gahlmann, A. Computational correction of spatially variant optical aberrations in 3D single-molecule localization microscopy. Opt. Express 27, 12582 (2019).
Möckl, L., Roy, A. R. & Moerner, W. E. Deep learning in single-molecule microscopy: fundamentals, caveats, and recent developments. Biomed. Opt. Express 11, 1633 (2020).
Nehme, E. et al. DeepSTORM3D: dense 3D localization microscopy and PSF design by deep learning. Nat. Methods 17, 734–740 (2020).
Speiser, A. et al. Deep learning enables fast and dense single-molecule localization with high accuracy. Nat. Methods 18, 1082–1090 (2021).
Kim, T., Moon, S. & Xu, K. Information-rich localization microscopy through machine learning. Nat. Commun. 10, 1996 (2019).
Nehme, E. et al. Learning optimal wavefront shaping for multi-channel imaging. IEEE Trans. Pattern Anal. Mach. Intell. 43, 2179–2192 (2021).
Zhang, P. et al. Analyzing complex single-molecule emission patterns with deep learning. Nat. Methods 15, 913–916 (2018).
Islam, M. A., Jia, S. & Bruce, N. D. B. How much position information do convolutional neural networks encode? ? In Proceedings of International Conference on Learning Representations (ICLR, 2020).
Liu, R. et al. in Advances in Neural Information Processing Systems (NeurIPS), Vol. 31, 9605–9616 (Curran Associates, 2018).
Siemons, M., Hulleman, C. N., Thorsen, R. Ø., Smith, C. S. & Stallinga, S. High-precision wavefront control in point spread function engineering for single emitter localization. Opt. Express 26, 8397 (2018).
Ferdman, B. et al. VIPR: vectorial implementation of phase retrieval for fast and accurate microscopic pixel-wise pupil estimation. Opt. Express 28, 10179 (2020).
Leutenegger, M., Rao, R., Leitgeb, R. A. & Lasser, T. Fast focus field calculations. Opt. Express 14, 4897–4903 (2006).
Li, Y. et al. Global fitting for high-accuracy multi-channel single-molecule localization. Nat. Commun. 13, 3133 (2022).
Möckl, L., Roy, A. R., Petrov, P. N. & Moerner, W. E. Accurate and rapid background estimation in single-molecule localization microscopy using the deep neural network BGnet. Proc. Natl Acad. Sci. USA 117, 60–67 (2020).
Novák, T., Gajdos, T., Sinkó, J., Szabó, G. & Erdélyi, M. TestSTORM: versatile simulator software for multimodal super-resolution localization fluorescence microscopy. Sci. Rep. 7, 951 (2017).
Sage, D. et al. Super-resolution fight club: assessment of 2D and 3D single-molecule localization microscopy software. Nat. Methods 16, 387–395 (2019).
Li, Y. et al. Real-time 3D single-molecule localization using experimental point spread functions. Nat. Methods 15, 367–369 (2018).
Ries, J. SMAP: a modular super-resolution microscopy analysis platform for SMLM data. Nat. Methods 17, 870–872 (2020).
Thevathasan, J. V. et al. Nuclear pores as versatile reference standards for quantitative superresolution microscopy. Nat. Methods 16, 1045–1053 (2019).
Pavani, S. R. P. et al. Three-dimensional, single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function. Proc. Natl Acad. Sci. USA 106, 2995–2999 (2009).
Shechtman, Y., Sahl, S. J., Backer, A. S. & Moerner, W. E. Optimal point spread function design for 3D imaging. Phys. Rev. Lett. 113, 133902 (2014).
Fu, S. et al. Deformable mirror-based optimal PSF engineering for 3D super-resolution imaging. Opt. Lett. 47, 3031 (2022).
Chaudhry, A., Shi, R. & Luciani, D. S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 318, E87–E101 (2020).
Arthur, D. & Vassilvitskii, S. in Proceedings of the Annual ACM-SIAM Symposium on Discrete Algorithms, 1027–1035 (SODA, 2007).
Xu, K., Zhong, G. & Zhuang, X. Actin, Spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339, 452–456 (2013).
Yanny, K., Monakhova, K., Shuai, R. W. & Waller, L. Deep learning for fast spatially varying deconvolution. Optica 9, 96 (2022).
Xu, F. et al. Three-dimensional nanoscopy of whole cells and tissues with in situ point spread function retrieval. Nat. Methods 17, 531–540 (2020).
El Beheiry, M. & Dahan, M. ViSP: representing single-particle localizations in three dimensions. Nat. Methods 10, 689–690 (2013).
Zhang, B., Zerubia, J. & Olivo-Marin, J.-C. Gaussian approximations of fluorescence microscope point-spread function models. Appl. Opt. 46, 1819 (2007).
Ronneberger, O. et al. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015, Vol. 9351, 234–241 (Springer, 2015).
Annibale, P., Vanni, S., Scarselli, M., Rothlisberger, U. & Radenovic, A. Quantitative photo-activated localization microscopy: unraveling the effects of photoblinking. PLoS ONE 6, e22678 (2011).
Perlin, K. Improving noise. ACM Trans. Graph. 21, 681–682 (2002).
Schröder, D., Deschamps, J., Dasgupta, A., Matti, U. & Ries, J. Cost-efficient open source laser engine for microscopy. Biomed. Opt. Express 11, 609 (2020).
Deschamps, J. & Ries, J. EMU: reconfigurable graphical user interfaces for Micro-Manager. BMC Bioinform. 21, 456 (2020).
Zhang, Y. et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013).
Thoma, E. C. et al. Ectopic expression of neurogenin 2 alone is sufficient to induce differentiation of embryonic stem cells into mature neurons. PLoS ONE 7, e38651 (2012).
Zhang, Y. et al. Nanoscale subcellular architecture revealed by multicolor three-dimensional salvaged fluorescence imaging. Nat. Methods 17, 225–231 (2020).
Zhou, R. et al. Proteomic and functional analyses of the periodic membrane skeleton in neurons. Nat. Commun. 13, 3196 (2022).
Acknowledgements
This work was supported by the Key Technology Research and Development Program of Shandong (2021CXGC010212), Guangdong Natural Science Foundation Joint Fund (2020A1515110380), Shenzhen Science and Technology Innovation Commission (grant nos. KQTD20200820113012029 and KQTD20210811090115021), Guangdong Provincial Key Laboratory of Advanced Biomaterials (2022B1212010003), China National Postdoctoral Program for Innovative Talents (BX20220141) and a startup grant from Southern University of Science and Technology.
Author information
Authors and Affiliations
Contributions
Y.L. conceived the concept and supervised the project. S.F. and W.S. developed the deep-learning-based algorithms. Y.L. and T.L. developed the GPU-based vectorial PSF fitter. W.S. developed the field-dependent PSF calibration software. J.Y., Y.L., Y.H., Z.H., Z.Y. and Z.G. designed the optical setup. W.S., L.Z., C.Y. and C.L. prepared the U2OS and COS-7 cell samples. J.L., X.L. and M.Z. contributed the induced neuronal cells from mESCs. W.S. and L.Z. labeled the cells and acquired the data. Y.H. and S.W. set up the microscope hardware. S.F., S.W. and T.L. analyzed the data. Y.L., S.F., W.S., J.R., P.X. and D.J. wrote the manuscript with the input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Thanh-An Pham and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Uniform illumination of large FOV.
a, Full frame (1608 ×1608 pixels) imaging of BG-AF647 dye solution (S9136S, New England Biolabs, 1:1000 dilution from stock in Milli-Q water) on coverslip. b, Intensity profile of the yellow dashed line in a. c, Example image of beads (T7279, Invitrogen, TetraSpeck) on coverslip (defocused by 300 nm) under uniform illumination. d, Zoomed image of the yellow rectangle indicated in c. Representative results are shown from 7 experiments. Scale bars, 20μm.
Extended Data Fig. 2 Comparison of experimental and fitted PSF models across the FOV.
Experiment and fitted astigmatism PSF models (a-e) and DMO Tetrapod PSF models (f-j) acquired from different positions in the full frame image (1608 × 1608 pixels). a1, Fitted pupil function of bead stacks locate at the left top position (79, 136). a2, Comparison of the experimental and fitted PSF model within ± 600 nm axial range. b-e are the same as a, but with different locations: b right top (1417, 22); c left bottom (246, 1588); d right bottom (1439, 1503); e middle (812, 805). f-j are experiment and fitted DMO Tetrapod PSF models within ± 3 μm axial range acquired from different positions: f left top (139, 75); g right top (1329, 206); h left bottom (177, 1422); i right bottom (1444, 1577); j middle (855, 810). Scale bars, 1 µm.
Extended Data Fig. 3 FD-DeepLoc architecture.
a, Overall network architecture of FD-DeepLoc. Similar to the DECODE network, FD-DeepLoc contains two U-Nets as indicated by the gray box. For each input frame, features will be extracted by the frame analysis module and then concatenated together to the temporal context module. Finally, a ten-channel map will be output to form the emitter predictions of each frame. The output of each layer is depicted with colorful blocks, where h and w represent the height and width of the channels. b, CoordConv channels. The CoordConv layer is placed in the first convolution layer of each module and has two convolution operations. One convolution (3×3) is operated on the input image. The other one (1×1) is operated on two coordinate channels. The results are then added as the output of the Coordconv layer.
Extended Data Fig. 4 Effects of PSF model mismatch between training and test dataset.
The training dataset is generated by PSF model with astigmatism aberration. Reconstructed images for test data using PSF models with astigmatism aberration (a, b, c), astigmatism plus spherical aberration (d, e, f) and astigmatism plus spherical and coma aberration (g, h, i) are shown. The performance of network with different loss function are compared: original DECODE loss function (a, d, g), original DECODE loss function plus cross entropy term (b, e, h) and original DECODE loss function plus cross entropy term and robust training (c, f, i). For robust training, small random aberrations were added (Methods). The signal to background ratio for test data is set as 5,500 average photons per emitter (sampled from a uniform distribution within [1,000, 10,000]) and 50 background photons per pixel. The rms wavefront error of spherical aberration and coma aberration are both set as 20 nm (λ = 660 nm). The ground truth coordinates are taken from the SMLM challenge training dataset MT029. Scale bar, 2 µm.
Extended Data Fig. 5 Comparison of localization accuracy for single molecules with field dependent aberrations analyzed by FD-DeepLoc and DECODE.
a, Localization accuracy of a left top single molecule at different axial positions analyzed by FD-DeepLoc and DECODE. Both networks were trained using the same training data containing all spatially variant PSFs. The bottom panel of a shows the corresponding 3D PSF. b-e are the same as a, but at different locations: b right top; c left bottom; d right bottom; e middle. The data is simulated using the aberration maps in Supplementary Fig. 6. 5,000 photons and 50 background were used for each single molecule. 3,000 single-emitter images are generated with random x, y positions at each axial position (x, y is random within a pixel, z is random within a 50 nm step, r.m.s.e. is averaged over 50 nm bins).
Extended Data Fig. 6 FD-DeepLoc enables large FOV 3D super-resolution imaging of nuclear pores with high fidelity.
a, Overview of the panoramic 3D super-resolution image of Nup96-SNAP-Alxea Fluor 647. b, Top views of the NPCs reconstructed with FD-DeepLoc, DECODE and Cspline. b1, Zoomed view of the region indicated by the white dashed box in a. b2, b3 and b4 are the zoomed images of the rectangle region indicated in b1 reconstructed by FD-DeepLoc, DECODE and Cspline separately. c, Side view images of the region bounded by the 500 nm dashed lines in b1 reconstructed by FD-DeepLoc, Cspline and DECODE separately. Representative results are shown from 3 experiments. Scale bars, 50 μm (a), 5 μm (b1,) and 1 μm (b2, c1).
Extended Data Fig. 7 Performance of FD-DeepLoc on 3D astigmatism imaging of mitochondria in a large FOV.
a, Overview of the panoramic 3D super-resolution image of immunolabeled TOM 20 in mitochondria. b1, c1, Zoomed images of the center and marginal areas as indicated by the dashed boxes b, c in a. b2, b3, Side-view cross-section of the region bounded by the dashed lines in b1 reconstructed by FD-DeepLoc and Cspline, separately. c2, c3 the same as b2, b3, but for the region bounded by the dashed lines in c1. Representative results are shown from 2 experiments. Scale bars, 50 μm (a), 10 μm (b1, c1) and 1 μm (b2, c2).
Extended Data Fig. 8 FD-DeepLoc enables large FOV whole cell 3D super-resolution imaging of NPC.
a, Overview of the panoramic whole cell 3D super-resolution image of NPC. b, Different axial sections of a. b1-3 are top views of axial range of (0 μm–2 μm), (2 μm–3 μm) and (3 μm – 5 μm), respectively. c, Magnified views of areas denoted by box c in a. c1, Top view of the box c area denoted in a. c2, Side-view cross-section of region denoted by the dashed line in c1. c3-4 are zoomed views of the top and bottom surface of the boxed area denoted in c1. d, Same as c for magnified views of region denoted by box d in a. e, The distribution of the fitted radii of the NPCs for the cell in c. e1 and e2 are for the top and bottom nucleus surface, respectively. f is the same as e, but for the cell in d. Representative results are shown from 7 experiments. Scale bars, 50 μm (a, b1), 5 μm (c1, c2, d1, d2) and 1 μm (c3, d3).
Extended Data Fig. 9 Quantitative analysis of whole cell 3D super-resolved mitochondria images.
a, Overview of 16 full frame images of whole cell 3D super-resolved mitochondria. b, Magnified angled views of 3 representative cells denoted by the boxes in a. c, Quantitative analysis of mitochondria morphology and network connectivity using Mitochondria Analyzer36. n = 121 cells imaged from 16 independent experiments and then classified by k-means++ into 3 groups. The numbers of cells for type 1/2/3 are 47/22/52, respectively. One-way ANOVA with two-sided Bonferroni multiple comparison test, the significance level was set as 0.05. The adjusted P-value for pairwise comparison is shown in each sub-panel of c. Boxplots are drawn from the 25th to 75th percentile with the horizontal line denoted the median and the whiskers extending to the minimum and maximum. Values outside 1.5 times interquartile range are defined as outliers which are shown as magenta circle. d, Principal component analysis was performed on the features used in c with the first 3 principal components plotted, color indicates the categorization results. Scale bars, 50 μm (a) and 10 μm (b1).
Extended Data Fig. 10 Layout of custom-built microscope used for this study.
M: mirror, DiM: dichroic mirror, FM: flip mirror, L: lens, TS: translation stage, FC: fiber coupler, MM fiber: multi-mode fiber, SM fiber: single-mode fiber, AP: aperture, BFP: back focal plane, FW: filter-wheel, TBL: tube lens, QPD: quadrant photodiode, DM: deformable mirror. a, Optical path of the microscope. Our setup has two excitation options: single-mode (SM) excitation and multi-mode (MM) excitation. In this work, we mainly use the MM mode for large FOV excitation. Excitation lasers are firstly reflected by dichroic mirrors DiM1-DiM3 (DMLP 425/505/605, Thorlabs) and then coupled by lens L1 into a multi-mode fiber (WFRCT200x200-230×230-440-620-1100 N, NA = 0.22, CeramOptec). The fiber is bound with a vibrator to generate a homogeneous illumination8,46. There are two imaging paths for the fluorescence detection which is separated by a flip mirror FM3. In the reflection path of FM3 (KSHM90, Owis), cylindrical lens (LJ1516L1-A, Thorlabs) based astigmatism 3D super-resolution imaging was performed. For the other imaging path without FM3, DM engineered PSFs were used for 3D super-resolution imaging. The focal length for each lens: L1 (f = 19 mm, Ø0.5 inch), L2 (f = 75 mm, Ø1 inch), L3 (PLN 10X/NA0.25, Olympus), L4(f = 400 mm, Ø1 inch), L5 (f = 35 mm, Ø1 inch), L6 (f = 400 mm, Ø75 mm), L7 (f = 400 mm, Ø75 mm), TBL (f = 180 mm, TTL180-A, Thorlabs), L8 (f = 150 mm, Ø2 inch), L9 (f = 150 mm, Ø2 inch), L10 (f = 75 mm, Ø1 inch), L11 (f = 150 mm, Ø2 inch), L12 (f = 150 mm, Ø2 inch), L13 (f = 1000 mm, LJ1516L1-A, Thorlabs). b, The rendered mechanical design using SolidWorks (Dassault Systèmes). Inset is the ray-tracing diagram of the imaging path rendered in OpticStudio (Zemax).
Supplementary information
Supplementary Information
Supplementary Figs. 1–14 and Supplementary Notes 1–6.
A tour view animation of the large-FOV 3D super-resolution image of NPC in Fig. 4.
A tour view animation of the large-FOV 3D super-resolution image of mitochondria in Extended Data Fig. 7.
Panoramic view animation of the large-FOV and DOF 3D super-resolution image of mitochondria in Fig. 5.
Animation of a single cell in the large-FOV and DOF 3D super-resolution image of mitochondria in Fig. 5.
Panoramic view animation of the large-FOV and DOF 3D super-resolution image of NPC in Extended Data Fig. 8.
Animation of a single cell in the large-FOV and DOF 3D super-resolution image of NPC in Extended Data Fig. 8.
A tour view animation of the large-FOV and DOF 3D super-resolution image of neurites in Fig. 6.
Supplementary Code 1
Example and source code for FD-DeepLoc.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 8
Statistical Source Data.
Source Data Extended Data Fig. 9
Statistical Source Data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, S., Shi, W., Luo, T. et al. Field-dependent deep learning enables high-throughput whole-cell 3D super-resolution imaging. Nat Methods 20, 459–468 (2023). https://doi.org/10.1038/s41592-023-01775-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-023-01775-5
This article is cited by
-
Optimization of table tennis target detection algorithm guided by multi-scale feature fusion of deep learning
Scientific Reports (2024)
-
SpiDe-Sr: blind super-resolution network for precise cell segmentation and clustering in spatial proteomics imaging
Nature Communications (2024)
-
The multimodality cell segmentation challenge: toward universal solutions
Nature Methods (2024)