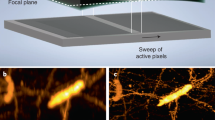
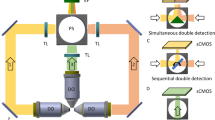
Abstract
Fluorescence microscopy has evolved from a purely observational tool to a platform for quantitative, hypothesis-driven research. As such, the demand for faster and less phototoxic imaging modalities has spurred a rapid growth in light sheet fluorescence microscopy (LSFM). By restricting the excitation to a thin plane, LSFM reduces the overall light dose to a specimen while simultaneously improving image contrast. However, the defining characteristics of light sheet microscopes subsequently warrant unique considerations in their use for quantitative experiments. In this Perspective, we outline many of the pitfalls in LSFM that can compromise analysis and confound interpretation. Moreover, we offer guidance in addressing these caveats when possible. In doing so, we hope to provide a useful resource for life scientists seeking to adopt LSFM to quantitatively address complex biological hypotheses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data used in this article are available at https://doi.org/10.6084/m9.figshare.c.6211429 or from the authors on request.
References
Wait, E. C., Reiche, M. A. & Chew, T. L. Hypothesis-driven quantitative fluorescence microscopy—the importance of reverse-thinking in experimental design. J. Cell Sci. 133, jcs250027 (2020).
Esposito, A. et al. Quantitative fluorescence microscopy techniques. Methods Mol. Biol. 586, 117–142 (2009).
Waters, J. C. & Wittmann, T. in Quantitative Imaging in Cell Biology (eds Waters, J. C. & Wittman, T.) Ch. 1 (Academic Press, 2014).
Lecoq, J., Orlova, N. & Grewe, B. F. Wide. Fast. Deep: recent advances in multiphoton microscopy of in vivo neuronal activity. J. Neurosci. 39, 9042–9052 (2019).
Stelzer, E. H. K. et al. Light sheet fluorescence microscopy. Nat. Rev. Methods Prim. 1, 73 (2021).
Jacquemet, G., Carisey, A. F., Hamidi, H., Henriques, R. & Leterrier, C. The cell biologist’s guide to super-resolution microscopy. J. Cell Sci. 133, jcs240713 (2020).
Ji, N. Adaptive optical fluorescence microscopy. Nat. Methods 14, 374–380 (2017).
Grimm, J. B. & Lavis, L. D. Caveat fluorophore: an insiders’ guide to small-molecule fluorescent labels. Nat. Methods https://doi.org/10.1038/s41592-021-01338-6 (2021).
Rodriguez, E. A. et al. The growing and glowing toolbox of fluorescent and photoactive proteins. Trends Biochem. Sci. 42, 111–129 (2017).
Lelek, M. et al. Single-molecule localization microscopy. Nat. Rev. Methods Prim. 1, 39 (2021).
Combs, C. A. & Shroff, H. Fluorescence microscopy: a concise guide to current imaging methods. Curr. Protoc. Neurosci. 2017, 2.1.1–2.1.25 (2017).
Reynaud, E. G., Peychl, J., Huisken, J. & Tomancak, P. Guide to light-sheet microscopy for adventurous biologists. Nat. Methods 12, 30–34 (2014).
Royer, L. A., Lemon, W. C., Chhetri, R. K. & Keller, P. J. A practical guide to adaptive light-sheet microscopy. Nat. Protoc. 13, 2462–2500 (2018).
Power, R. M. & Huisken, J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat. Methods 14, 360–373 (2017).
Girkin, J. M. & Carvalho, M. T. The light-sheet microscopy revolution. J. Optics 20, 053002 (2018).
Wan, Y., McDole, K. & Keller, P. J. Light-sheet microscopy and its potential for understanding developmental processes. Annu. Rev. Cell Dev. Biol. 35, 655–681 (2019).
Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J. & Stelzer, E. H. K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004).
Voie, A. H., Burns, D. H. & Spelman, F. A. Orthogonal‐plane fluorescence optical sectioning: three‐dimensional imaging of macroscopic biological specimens. J. Microsc. 170, 229–236 (1993).
Gebhardt, J. C. M. et al. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods 10, 421–426 (2013).
Wolf, S. et al. Whole-brain functional imaging with two-photon light-sheet microscopy. Nat. Methods 12, 379–380 (2015).
Waters, J. C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 185, 1135–1148 (2009).
Jonkman, J., Brown, C. M., Wright, G. D., Anderson, K. I. & North, A. J. Tutorial: guidance for quantitative confocal microscopy. Nat. Protoc. 15, 1585–1611 (2020).
North, A. J. Seeing is believing? A beginners’ guide to practical pitfalls in image acquisition. J. Cell Biol. 172, 9–18 (2006).
Lee, J. Y. & Kitaoka, M. A beginner’s guide to rigor and reproducibility in fluorescence imaging experiments. Mol. Biol. Cell 29, 1519–1525 (2018).
Brown, C. M. Fluorescence microscopy—avoiding the pitfalls. J. Cell Sci. 120, 1703–1705 (2007).
Chen, B. C. et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
Keller, P. J., Schmidt, A. D., Wittbrodt, J. & Stelzer, E. H. K. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008).
Chang, B. J. et al. Universal light-sheet generation with field synthesis. Nat. Methods 16, 235–238 (2019).
Self, S. A. Focusing of spherical Gaussian beams. Appl. Opt. 22, 658 (1983).
Planchon, T. A. et al. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nat. Methods 8, 417–423 (2011).
Fahrbach, F. O. & Rohrbach, A. A line scanned light-sheet microscope with phase shaped self-reconstructing beams. Opt. Express 18, 24229 (2010).
Gao, L. Extend the field of view of selective plan illumination microscopy by tiling the excitation light sheet. Opt. Express https://doi.org/10.1364/oe.23.006102 (2015).
Dean, K. M., Roudot, P., Welf, E. S., Danuser, G. & Fiolka, R. Deconvolution-free subcellular imaging with axially swept light sheet microscopy. Biophys. J. 108, 2807–2815 (2015).
Kim, B. et al. Open-top axially swept light-sheet microscopy. Biomed. Opt. Express 12, 2328 (2021).
Liu, Y., Rollins, A. M. & Jenkins, M. W. CompassLSM: axially swept light-sheet microscopy made simple. Biomed. Opt. Express 12, 6571 (2021).
Landry, J., Hamann, S. & Solgaard, O. High-speed axially swept light sheet microscopy using a linear MEMS phased array for isotropic resolution. J. Biomed. Opt. 25, 106504 (2020).
Chakraborty, T. et al. Light-sheet microscopy of cleared tissues with isotropic, subcellular resolution. Nat. Methods https://doi.org/10.1038/s41592-019-0615-4 (2019).
Wu, Y. et al. Spatially isotropic four-dimensional imaging with dual-view plane illumination microscopy. Nat. Biotechnol. 31, 1032–1038 (2013).
Kumar, A. et al. Dual-view plane illumination microscopy for rapid and spatially isotropic imaging. Nat. Protoc. 9, 2555–2573 (2014).
Octave, J. ‐N., Schneider, Y. ‐J., Trouet, A. & Crichton, R. R. Transferrin uptake by cultured rat embryo fibroblasts: the influence of temperature and incubation time, subcellular distribution and short‐term kinetic studies. Eur. J. Biochem. 115, 611–618 (1981).
Tsien, R. Y., Ernst, L. & Waggoner, A. in Handbook of Biological Confocal Microscopy 3rd edn (ed. Pawley, J. B.) 338–352 (Springer, 2006).
Gavryusev, V. et al. Dual-beam confocal light-sheet microscopy via flexible acousto-optic deflector. J. Biomed. Opt. 24, 106504 (2019).
Glaser, A. K. et al. Multidirectional digital scanned light-sheet microscopy enables uniform fluorescence excitation and contrast-enhanced imaging. Sci. Rep. 8, 13878 (2018).
G. De, Medeiros et al. Confocal multiview light-sheet microscopy. Nat. Commun. 6, 8881 (2015).
Baumgart, E. & Kubitscheck, U. Scanned light sheet microscopy with confocal slit detection. Opt. Express 20, 21805 (2012).
Chakraborty, T. et al. Light-sheet microscopy of cleared tissues with isotropic, subcellular resolution. Nat. Methods 16, 1109–1113 (2019).
Shutova, M. S. & Svitkina, T. M. Common and specific functions of nonmuscle myosin II paralogs in cells. Biochemistry (Mosc.) https://doi.org/10.1134/S0006297918120040 (2018).
Kask, P., Palo, K., Hinnah, C. & Pommerencke, T. Flat field correction for high-throughput imaging of fluorescent samples. J. Microsc. https://doi.org/10.1111/jmi.12404 (2016).
Smith, K. et al. CIDRE: an illumination-correction method for optical microscopy. Nat. Methods https://doi.org/10.1038/nmeth.3323 (2015).
Likar, B., Maintz, J. B. A., Viergever, M. A. & Pernuš, F. Retrospective shading correction based on entropy minimization. J. Microsc. https://doi.org/10.1046/j.1365-2818.2000.00669.x (2000).
Tomer, R., Khairy, K., Amat, F. & Keller, P. J. Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy. Nat. Methods 9, 755–763 (2012).
Huisken, J. & Stainier, D. Y. R. Even fluorescence excitation by multidirectional selective plane illumination microscopy (mSPIM). Opt. Lett. 32, 2608 (2007).
Ricci, P. et al. Removing striping artifacts in light-sheet fluorescence microscopy: a review. Prog. Biophys. Mol. Biol. 168, 52–65 (2022).
Gao, R. et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution. Science 363, eaau8302 (2019).
Guo, M. et al. Rapid image deconvolution and multiview fusion for optical microscopy. Nat. Biotechnol. 38, 1337–1346 (2020).
Ueda, H. R. et al. Whole-brain profiling of cells and circuits in mammals by tissue clearing and light-sheet microscopy. Neuron 106, 369–387 (2020).
Voigt, F. F. et al. The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue. Nat. Methods 16, 1105–1108 (2019).
Chen, F., Tillberg, P. W. & Boyden, E. S. Expansion microscopy. Science 347, 543–548 (2015).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Dodt, H. U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 (2007).
Hama, H. et al. ScaleS: an optical clearing palette for biological imaging. Nat. Neurosci. 18, 1518–1529 (2015).
Jing, D. et al. Tissue clearing of both hard and soft tissue organs with the pegasos method. Cell Res. 28, 803–818 (2018).
Renier, N. et al. IDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014).
Susaki, E. A. et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell 157, 726–739 (2014).
Tainaka, K., Kuno, A., Kubota, S. I., Murakami, T. & Ueda, H. R. Chemical principles in tissue clearing and staining protocols for whole-body cell profiling. Annu. Rev. Cell Dev. Biol. 32, 713–741 (2016).
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009).
Burger, W. & Burge, M. Principles of Digital Image Processing: Advanced Methods. Principles of Digital Image Processing (Springer, 2013).
Pitas, I. Digital Image Processing Algorithms and Applications (Wiley, 2000).
Wallace, W., Schaefer, L. H. & Swedlow, J. R. A workingperson’s guide to deconvolution in light microscopy. BioTechniques https://doi.org/10.2144/01315bi01 (2001).
Biggs, D. S. C. A practical guide to deconvolution of fluorescence microscope imagery. Micros. Today https://doi.org/10.1017/s1551929510991311 (2010).
McNally, J. G., Karpova, T., Cooper, J. & Conchello, J. A. Three-dimensional imaging by deconvolution microscopy. Methods https://doi.org/10.1006/meth.1999.0873 (1999).
Aaron, J. & Chew, T. L. A guide to accurate reporting in digital image processing—can anyone reproduce your quantitative analysis? J. Cell Sci. https://doi.org/10.1242/jcs.254151 (2021).
Pitrone, P. G. et al. OpenSPIM: an open-access light-sheet microscopy platform. Nat. Methods 10, 598–599 (2013).
Swoger, J., Verveer, P., Greger, K., Huisken, J. & Stelzer, E. H. K. Multi-view image fusion improves resolution in three-dimensional microscopy. Opt. Express 15, 8029 (2007).
Krzic, U., Gunther, S., Saunders, T. E., Streichan, S. J. & Hufnagel, L. Multiview light-sheet microscope for rapid in toto imaging. Nat. Methods 9, 730–733 (2012).
Chhetri, R. K. et al. Whole-animal functional and developmental imaging with isotropic spatial resolution. Nat. Methods 12, 1171–1178 (2015).
Preibisch, S., Saalfeld, S., Schindelin, J. & Tomancak, P. Software for bead-based registration of selective plane illumination microscopy data. Nat. Methods 7, 418–419 (2010).
Amat, F. et al. Efficient processing and analysis of large-scale light-sheet microscopy data. Nat. Protoc. 10, 1679–1696 (2015).
Preibisch, S. et al. Efficient Bayesian-based multiview deconvolution. Nat. Methods 11, 645–648 (2014).
Brown, L. G. A survey of image registration techniques. ACM Comput. Surv. 24, 325–376 (1992).
Ashburner, J. & Friston, K. in Human Brain Function 2nd edn (eds Friston, K. et al.) 635–653 (Elsevier, 2003).
Ruthotto, L. & Modersitzki, J. in Handbook of Mathematical Methods in Imaging 2nd edn, Vol. 1 (ed. Scherzer, O.) 2005–2051 (Springer, 2015).
Wu, Y. et al. Reflective imaging improves spatiotemporal resolution and collection efficiency in light sheet microscopy. Nat. Commun. 8, 1452 (2017).
Allais, M. L’anisotropie de l’espace: la nécessaire révision de certains postulats des théories contemporaines. Les données de l’expérience (Clément Juglar, 1997).
Royer, L. A. et al. Adaptive light-sheet microscopy for long-term, high-resolution imaging in living organisms. Nat. Biotechnol. 34, 1267–1278 (2016).
Siedentopf, H. & Zsigmondy, R. Uber Sichtbarmachung und Größenbestimmung ultramikoskopischer Teilchen, mit besonderer Anwendung auf Goldrubingläser. Ann. Phys. 315, 1–39 (1902).
Chew, T.-L., George, R., Soell, A. & Betzig, E. Opening a path to commercialization. Opt. Photonics N. 28, 42 (2017).
Reiche, M. A. et al. When light meets biology: how the specimen affects quantitative microscopy. J. Cell Sci. 135, jcs259656 (2022).
Hampson, K. M. et al. Adaptive optics for high-resolution imaging. Nat. Rev. Methods Prim. 1, 68 (2021).
Liu, T. L. et al. Observing the cell in its native state: imaging subcellular dynamics in multicellular organisms. Science 360, eaaq1392 (2018).
Hubert, A. et al. Adaptive optics light-sheet microscopy based on direct wavefront sensing without any guide star. Opt. Lett. 44, 2514 (2019).
Wilding, D., Pozzi, P., Soloviev, O., Vdovin, G. & Verhaegen, M. Adaptive illumination based on direct wavefront sensing in a light-sheet fluorescence microscope. Opt. Express 24, 24896 (2016).
Bourgenot, C., Saunter, C. D., Taylor, J. M., Girkin, J. M. & Love, G. D. 3D adaptive optics in a light sheet microscope. Opt. Express 20, 13252 (2012).
Schoeneberg, J. 4D cell biology: adaptive optics lattice light-sheet imaging and AI powered big data processing of live stem cell-derived organoids. J. Biomol. Tech. 31, S33 (2020).
Mahou, P., Vermot, J., Beaurepaire, E. & Supatto, W. Multicolor two-photon light-sheet microscopy. Nat. Methods 11, 600–601 (2014).
Truong, T. V., Supatto, W., Koos, D. S., Choi, J. M. & Fraser, S. E. Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nat. Methods 8, 757–762 (2011).
Zong, W. et al. Large-field high-resolution two-photon digital scanned light-sheet microscopy. Cell Res. 25, 254–257 (2015).
Escobet-Montalbán, A. et al. Three-photon light-sheet fluorescence microscopy. Opt. Lett. 43, 5484 (2018).
Weigert, M. et al. Content-aware image restoration: pushing the limits of fluorescence microscopy. Nat. Methods 15, 1090–1097 (2018).
Krull, A., Vičar, T., Prakash, M., Lalit, M. & Jug, F. Probabilistic Noise2Void: unsupervised content-aware denoising. Front. Comput. Sci. https://doi.org/10.3389/fcomp.2020.00005 (2020).
Acknowledgements
We thank S. Khuon, L. Eisenman, A. Nain, C. Hernandez-Padillaand and L. Zhang for cell culture and sample preparation; W. Lemon for preparation of D. melanogaster embryos; D. Dalle Nogare and A. Chitnis for preparation of zebrafish samples; R. Christensen for C. elegans sample preparation; Z. Bao for providing the OD58 C. elegans strain; Y. Su, I. Curtin, F. Kyere, J. Stein and M.S. Itano for advice and assistance with brain sample preparation; and M. DeSantis and the Janelia Light Microscopy Facility. This work was supported in part by the intramural research program of the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health (M.G., H.D.V., Y.W. and H.S.). The Advanced Imaging Center at Janelia Research Campus was generously supported by the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation (C.M.H. and T.-L.C.).
Author information
Authors and Affiliations
Contributions
C.M.H., M.G., H.D.V. and Y.W. performed the imaging experiments and accompanying analyses. H.S. and T.-L.C. oversaw the project. All authors contributed to writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Kevin Dean, Niall Geoghegan, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ‘Practical Considerations for Quantitative Light Sheet Fluorescence Microscopy’ Infographic.
Summary of the important considerations for quantitative LSFM described in this Perspective.
Supplementary information
Supplementary Information
Supplementary Methods and Figs. 1–8.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hobson, C.M., Guo, M., Vishwasrao, H.D. et al. Practical considerations for quantitative light sheet fluorescence microscopy. Nat Methods 19, 1538–1549 (2022). https://doi.org/10.1038/s41592-022-01632-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-022-01632-x
This article is cited by
-
Smart lattice light-sheet microscopy for imaging rare and complex cellular events
Nature Methods (2024)
-
Imagining the future of optical microscopy: everything, everywhere, all at once
Communications Biology (2023)