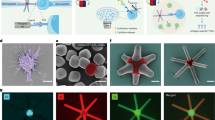
Abstract
Adaptive immunity relies on T lymphocytes that use αβ T cell receptors (TCRs) to discriminate among peptides presented by major histocompatibility complex molecules (pMHCs). Identifying pMHCs capable of inducing robust T cell responses will not only enable a deeper understanding of the mechanisms governing immune responses but could also have broad applications in diagnosis and treatment. T cell recognition of sparse antigenic pMHCs in vivo relies on biomechanical forces. However, in vitro screening methods test potential pMHCs without force and often at high (nonphysiological) pMHC densities and thus fail to predict potent agonists in vivo. Here, we present a technology termed BATTLES (biomechanically assisted T cell triggering for large-scale exogenous-pMHC screening) that uses biomechanical force to initiate T cell triggering for peptides and cells in parallel. BATTLES displays candidate pMHCs on spectrally encoded beads composed of a thermo-responsive polymer capable of applying shear loads to T cells, facilitating exploration of the force- and sequence-dependent landscape of T cell responses. BATTLES can be used to explore basic T cell mechanobiology and T cell-based immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data required to reproduce all figures have been deposited to an OSF repository (https://osf.io/xs7zf/). Source data are provided with this paper.
Code availability
AutoCAD designs (parallel flow focuser and microwell device) and T cell Ca2+ flux data have been deposited to an OSF repository (https://osf.io/xs7zf/).
References
Vanguri, V., Govern, C. C., Smith, R. & Huseby, E. S. Viral antigen density and confinement time regulate the reactivity pattern of CD4 T-cell responses to vaccinia virus infection. Proc. Natl Acad. Sci. USA 110, 288–293 (2013).
Huang, J. et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4+ T cells. Immunity 39, 846–857 (2013).
Purbhoo, M. A., Irvine, D. J., Huppa, J. B. & Davis, M. M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 5, 524–530 (2004).
Keskin, D. B. et al. Physical detection of influenza A epitopes identifies a stealth subset on human lung epithelium evading natural CD8 immunity. Proc. Natl Acad. Sci. USA 112, 2151–2156 (2015).
Sasada, T., Ghendler, Y., Wang, J.-H. & Reinherz, E. L. Thymic selection is influenced by subtle structural variation involving the p4 residue of an MHC class I-bound peptide. Eur. J. Immunol. 30, 1281–1289 (2000).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010).
Lyons, D. S. et al. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity 5, 53–61 (1996).
Stone, J. D., Chervin, A. S. & Kranz, D. M. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 126, 165–176 (2009).
Sibener, L. V. et al. Isolation of a structural mechanism for uncoupling T cell receptor signaling from peptide-MHC binding. Cell 174, 672–687.e627 (2018).
Ueno, T., Tomiyama, H., Fujiwara, M., Oka, S. & Takiguchi, M. Functionally impaired HIV-specific CD8 T cells show high affinity TCR-ligand interactions. J. Immunol. 173, 5451–5457 (2004).
Corse, E., Gottschalk, R. A., Krogsgaard, M. & Allison, J. P. Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol. 8, e1000481 (2010).
Feng, Y., Reinherz, E. L. & Lang, M. J. αβ T cell receptor mechanosensing forces out serial engagement. Trends Immunol. 39, 596–609 (2018).
Feng, Y. et al. Mechanosensing drives acuity of αβ T-cell recognition. Proc. Natl Acad. Sci. USA 114, E8204–E8213 (2017).
Liu, B., Chen, W., Evavold, B. D. & Zhu, C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell 157, 357–368 (2014).
Das, D. K. et al. Force-dependent transition in the T-cell receptor β-subunit allosterically regulates peptide discrimination and pMHC bond lifetime. Proc. Natl Acad. Sci. USA 112, 1517–1522 (2015).
Bashour, K. T. et al. CD28 and CD3 have complementary roles in T-cell traction forces. Proc. Natl Acad. Sci. USA 111, 2241–2246 (2014).
Basu, R. et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell 165, 100–110 (2016).
Hui, K. L., Balagopalan, L., Samelson, L. E. & Upadhyaya, A. Cytoskeletal forces during signaling activation in Jurkat T-cells. Mol. Biol. Cell 26, 685–695 (2015).
Dustin, M. L. & Groves, J. T. Receptor signaling clusters in the immune synapse. Annu. Rev. Biophys. 41, 543–556 (2012).
Liu, Y. et al. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proc. Natl Acad. Sci. USA 113, 5610–5615 (2016).
Wei, X., Tromberg, B. J. & Cahalan, M. D. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca(2+) signaling. Proc. Natl Acad. Sci. USA 96, 8471–8476 (1999).
Hu, K. H. & Butte, M. J. T cell activation requires force generation. J. Cell Biol. 213, 535–542 (2016).
Husson, J., Chemin, K., Bohineust, A., Hivroz, C. & Henry, N. Force generation upon T cell receptor engagement. PLoS ONE 6, e19680 (2011).
Li, Y.-C. et al. Cutting edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J. Immunol. 184, 5959–5963 (2010).
Liu, Z. et al. Nanoscale optomechanical actuators for controlling mechanotransduction in living cells. Nat. Methods 13, 143–146 (2016).
Riley, T. P. et al. T cell receptor cross-reactivity expanded by dramatic peptide–MHC adaptability. Nat. Chem. Biol. 14, 934–942 (2018).
Kim, J. & Hayward, R. C. Mimicking dynamic in vivo environments with stimuli-responsive materials for cell culture. Trends Biotechnol. 30, 426–439 (2012).
Chandorkar, Y. et al. Cellular responses to beating hydrogels to investigate mechanotransduction. Nat. Commun. 10, 4027 (2019).
Lau, A., Portigliatti, M., Raphaël, E. & Léger, L. Spreading of latex particles on a substrate. Europhys. Lett. 60, 717 (2002).
Ding, T. et al. Light-induced actuating nanotransducers. Proc. Natl Acad. Sci. USA 113, 5503–5507 (2016).
Burmistrova, A., Richter, M., Eisele, M., Üzüm, C. & Von Klitzing, R. The effect of co-monomer content on the swelling/shrinking and mechanical behaviour of individually adsorbed PNIPAM microgel particles. Polymers 3, 1575–1590 (2011).
Feng, Y., White, A. K., Hein, J. B., Appel, E. A. & Fordyce, P. M. MRBLES 2.0: high-throughput generation of chemically functionalized spectrally and magnetically encoded hydrogel beads using a simple single-layer microfluidic device. Microsyst. Nanoeng. 6, 109 (2020).
WoldeMussie, E., Maeyama, K. & Beaven, M. Loss of secretory response of rat basophilic leukemia (2H3) cells at 40 degrees C is associated with reversible suppression of inositol phospholipid breakdown and calcium signals. J. Immunol. 137, 1674–1680 (1986).
Thauland, T. J., Hu, K. H., Bruce, M. A. & Butte, M. J. Cytoskeletal adaptivity regulates T cell receptor signaling. Sci. Signal. 10, eaah3737 (2017).
Jung, P., Zhou, X., Iden, S., Bischoff, M. & Qu, B. T cell stiffness is enhanced upon formation of immunological synapse. eLife 10, e66643 (2021).
Judokusumo, E., Tabdanov, E., Kumari, S., Dustin, M. L. & Kam, L. C. Mechanosensing in T lymphocyte activation. Biophys. J. 102, L5–L7 (2012).
Wu, P.-H. et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 15, 491–498 (2018).
Meng, K. P., Majedi, F. S., Thauland, T. J. & Butte, M. J. Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med. 217, e20200053 (2020).
Gerver, R. E. et al. Programmable microfluidic synthesis of spectrally encoded microspheres. Lab Chip 12, 4716–4723 (2012).
Nguyen, H. Q. et al. Programmable microfluidic synthesis of over one thousand uniquely identifiable spectral codes. Adv. Opt. Mater. 5, 1600548 (2017).
Harink, B., Nguyen, H., Thorn, K. & Fordyce, P. An open-source software analysis package for Microspheres with Ratiometric Barcode Lanthanide Encoding (MRBLEs). PLoS ONE 14, e0203725 (2019).
Rodenko, B. et al. Generation of peptide–MHC class I complexes through UV-mediated ligand exchange. Nat. Protoc. 1, 1120 (2006).
Fritzsche, M. et al. Cytoskeletal actin dynamics shape a ramifying actin network underpinning immunological synapse formation. Sci. Adv. 3, e1603032 (2017).
Ma, Z., Sharp, K. A., Janmey, P. A. & Finkel, T. H. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 6, e43 (2008).
Freedman, M. Early biochemical events in lymphocyte activation: I. Investigations on the nature and significance of early calcium fluxes observed in mitogen-induced T and B lymphocytes. Cell. Immunol. 44, 290–313 (1979).
Kim, S. T. et al. The αβ T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 284, 31028–31037 (2009).
Brazin, K. N. et al. The T cell antigen receptor α transmembrane domain coordinates triggering through regulation of bilayer immersion and CD3 subunit associations. Immunity 49, 829–841. e826 (2018).
Della Sala, F. et al. Mechanical behavior of bioactive poly(ethylene glycol) diacrylate matrices for biomedical application. J. Mech. Behav. Biomed. Mater. 110, 103885 (2020).
Chen, X., Mao, Z. & Chen, B. Probing time-dependent mechanical behaviors of catch bonds based on two-state models. Sci. Rep. 5, 7868 (2015).
Chen, Y. et al. Fast force loading disrupts molecular binding stability in human and mouse cell adhesions. Mol. Cell. Biomech. 16, 211–223 (2019).
Pryshchep, S., Zarnitsyna, V. I., Hong, J., Evavold, B. D. & Zhu, C. Accumulation of serial forces on TCR and CD8 frequently applied by agonist antigenic peptides embedded in MHC molecules triggers calcium in T cells. J. Immunol. 193, 68–76 (2014).
Jin, Y. et al. Thermally tunable dynamic and static elastic properties of hydrogel due to volumetric phase transition. Polymers 12, 1462 (2020).
Henrickson, S. E. et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat. Immunol. 9, 282–291 (2008).
Chodon, T. et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin. Cancer Res. 20, 2457 (2014).
Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 17, 679–690 (2017).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Wülfing, C. & Davis, M. M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282, 2266–2269 (1998).
Lei, K., Kurum, A. & Tang, L. Mechanical immunoengineering of T cells for therapeutic applications. Acc. Chem. Res. 53, 2777–2790 (2020).
Ghosh, S. et al. ERM-Dependent assembly of T cell receptor signaling and co-stimulatory molecules on microvilli prior to activation. Cell Rep. 30, 3434–3447.e3436 (2020).
Das, D. K. et al. Pre-T cell receptors (pre-TCRs) leverage Vβ complementarity determining regions (CDRs) and hydrophobic patch in mechanosensing thymic self-ligands. J. Biol. Chem. 291, 25292–25305 (2016).
Christo, S. N. et al. Scrutinizing calcium flux oscillations in T lymphocytes to deduce the strength of stimulus. Sci. Rep. 5, 7760 (2015).
Bentzen, A. K. et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol. 34, 1037–1045 (2016).
Bentzen, A. K. et al. T cell receptor fingerprinting enables in-depth characterization of the interactions governing recognition of peptide–MHC complexes. Nat. Biotechnol. 36, 1191–1196 (2018).
Zhang, S.-Q. et al. High-throughput determination of the antigen specificities of T cell receptors in single cells. Nat. Biotechnol. 36, 1156–1159 (2018).
Birnbaum, M. E. et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell 157, 1073–1087 (2014).
Joglekar, A. V. et al. T cell antigen discovery via signaling and antigen-presenting bifunctional receptors. Nat. Methods 16, 191–198 (2019).
Kula, T. et al. T-scan: a genome-wide method for the systematic discovery of T cell epitopes. Cell 178, 1016–1028. e1013 (2019).
Li, G. et al. T cell antigen discovery via trogocytosis. Nat. Methods 16, 183–190 (2019).
Wolf, K., Müller, R., Borgmann, S., Bröcker, E.-B. & Friedl, P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3262–3269 (2003).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Harding, C. V. & Unanue, E. R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature 346, 574–576 (1990).
Demotz, S., Grey, H. M. & Sette, A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science 249, 1028–1030 (1990).
Kimachi, K., Croft, M. & Grey, H. M. The minimal number of antigen-major histocompatibility complex class II complexes required for activation of naive and primed T cells. Eur. J. Immunol. 27, 3310–3317 (1997).
Brower, R. et al. Minimal requirements for peptide mediated activation of CD8+ CTL. Mol. Immunol. 31, 1285–1293 (1994).
Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4, 565–571 (1996).
Gross, G., Gorochov, G., Waks, T. & Eshhar, Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplantation Proc. 21, 127–130 (1989).
Chen, Y., Ju, L., Rushdi, M., Ge, C. & Zhu, C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell 28, 3134–3155 (2017).
Acknowledgements
This work was supported by National Institutes of Health (NIH) grant nos. 1DP2GM123641 (awarded to P.M.F.) and R01GM107132, and a Stanford Bio-X Interdisciplinary Initiatives seed grant. K.C.G. is an investigator of the Howard Hughes Medical Institute, and is also supported by grant nos. NIH-5R01AI103867, U19AI057229, Mathers Foundation and Ludwig Foundation support. P.M.F. is a Chan Zuckerberg Biohub Investigator and acknowledges the support of a Sloan Research Foundation Fellowship. Y.F. is a Cancer Research Institute Postdoctoral Fellow. X.Z. is funded by a Stanford Bio-X seed grant. A.K.W. was funded by the Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship. Part of this work was performed at the Stanford Nano Shared Facilities, supported by the National Science Foundation under award no. ECCS-1542152. We also thank E. Appel and D. Chan for helpful discussions concerning polymers and help with shear modulus measurements and Z. Bryant and P.V. Ruijgrok for help with TIRF microscopy.
Author information
Authors and Affiliations
Contributions
Y.F. conceptualized the platform and validation experiments. X.Z. made all the T cell lines and generated pMHC complexes refolded with UV-labile peptides. Y.F., X.Z. and A.K.W. analyzed data. K.C.G. and P.M.F. provided funding, resources, mentorship and project supervision. Y.F., X.Z., A.K.W., K.C.G. and P.M.F. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Stanford University and Chan Zuckerberg Biohub have filed a provisional patent application (US Provisional Patent application no. 63/108,162) on the BATTLES technology described here, and Y.F., X.Z., A.K.W., K.C.G. and P.M.F. are named inventors.
Peer review
Peer review information
Nature Methods thanks Ellis L. Reinherz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Madhura Mukhopadhyay, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Generation of thermo-responsive beads using a microfluidic parallel flow focusing system.
(a) Bead generation setup that includes a PDMS parallel flow focusing device, a low-cost inverted microscope, and two syringe pumps for injection of aqueous and oil phases. Generated droplets were collected into a 24-well plate. (b) Images of droplet generation and collection at position 1 and position 2 within the PDMS device (left), with an average droplet radius of 16.1 ± 0.4 μm (right). (c) Image of thermo-responsive beads after UV polymerization of droplets and rehydration in PBST buffer (left), with an average bead radius of 23.75 ± 1.55 μm (right). Scale bars: 100 μm in b and 50 μm in c. Images from (b) to (c) represent 21 independent experiments.
Extended Data Fig. 2 Modulus of rigidity κ measurement using thermo-responsive slabs.
(a) Procedure to generate thermo-responsive slabs from an identical formulation as thermo-responsive beads followed by the measurement of κ by TA rheometer. (b) Measured storage modulus, loss modulus, and modulus of rigidity as a function of angular frequency; κ was determined from the root sum square of the storage modulus and loss modulus at each angular frequency and then averaged.
Extended Data Fig. 3 Calculated coding capacity for thermo-responsive beads.
(a) Matrix of Sm/Eu and Dy/Eu cluster variances as a function of Sm and Dy cluster medians, respectively. (b) Calculated target ratios for a thermo-responsive bead code set with 5-standard deviation spacing between cluster medians. (c) Number of calculated code clusters when requiring 3-, 4-, 5- and 6-standard deviation (STD) spacing between each code cluster.
Extended Data Fig. 4 Single molecule TIRF fluorescence imaging analysis of pMHC molecules immobilized on 12 thermo-responsive beads at the bead/glass interface.
(a) TIRF images (left) and integrated analysis (counts of bright punctae across all images, right) for a single bead from code 8 bearing biotinylated VPLTEDALL/B35 complexes. In each image, the white circle indicates the radius of the settled bead and the orange square indicates the region of interest used to estimate pMHC density. (b) Representative TIRF images (left) and integrated analysis (counts of bright punctae across all images, right) for a single negative control bead. In each experiment, biotinylated pMHC molecules were detected by incubation with PE-labeled anti-β2 mAb (clone: A17082A) immunofluorescence. Detailed bead preparation methods are found in Methods; the orange square ROI is ~840 μm2. We acquired images similar to those shown in (a) and (b) across 10 and 5 independent experiments, respectively. Scale bars: 50 µm.
Extended Data Fig. 5 Measured Ca2+-induced fluorescence as a function of time for TCR589-transduced T cells interacting with 21 different peptide sequences.
(a) All measured traces. (b) All positive traces with the indicated numbers of positive cells and measured cells and positive percentages. Light gray lines indicate traces for individual cells; dark grey lines indicate the mean signals. Light blue shade represents SEM.
Extended Data Fig. 6 Measured integrated Ca2+ fluorescence signals and estimated significance for TCR55 negative controls.
(a) Left: Integrated Ca2+ signals for each positive cell (light grey markers) and the mean integrated Ca2+ signal (light grey square) at 34 °C. Right: Estimated p-value vs. log2-transformed mean fold change for TCR55-tranduced T cells interacting with 21 different peptide sequences at 34 °C. Grey box indicates Bonferroni-corrected p-value at a significance of 0.05 (p = 0.0024). Correspondence between thermo-responsive bead spectral code and displayed peptide sequence is shown at right. n = 1320 cells in total. (b) Left: Integrated Ca2+ signals for each positive cell (light grey markers) and the mean integrated Ca2+ signal (light grey square) at 37 °C. Right: Estimated p-value vs. log2-transformed mean fold change for TCR55-tranduced T cells interacting with 21 different peptide sequences at 37 °C. Grey box indicates Bonferroni-corrected p-value at a significance of 0.05 (p = 0.0024). Correspondence between thermo-responsive bead spectral code and displayed peptide sequence is shown at right. n = 845 cells in total. The box width in (a) and (b) defines the interquartile range (IQR) and whiskers extend to 1.5 × IQR in either direction. p-values were calculated via two-sided bootstrapping as detailed in Methods. (c) Integrated Ca2+ signals for all positive cells (dark grey markers) at 34 °C, 37 °C and with expansion force applied (bars and error bars indicate mean and standard deviation, respectively; positive cell numbers and percentages are indicated above each bar.
Extended Data Fig. 7 Measured integrated Ca2+ fluorescence signals and estimated significance for TCR55 interacting with either: (1) PEG-DA beads (which do not change size upon a small temperature change) bearing the stimulatory peptide VPLTEDALL; (2) thermo-responsive NIPAM beads bearing the non-stimulatory peptide VPIVEDSFL; and (3) thermo-responsive NIPAM beads bearing the stimulatory peptide VPLTEDALL.
(a) Results at 34 °C alone, (b) results at 37 °C alone, and (c) results for heating to 37 °C and then cooling to 34 °C. Each panel shows: (left) integrated Ca2+ signals for each positive cell (light grey markers) and the mean integrated Ca2+ signal (light grey square) and (right) estimated p-value vs. log2-transformed mean fold change for TCR55-tranduced T cells interacting with each peptide-bead combination. Positive cell percentages are indicated above each bar. Grey box indicates Bonferroni-corrected p-value at a significance of 0.05 (p = 0.0167). Correspondence between embedded spectral code and displayed peptide sequence is shown at right. In all box plots, the box width defines the interquartile range (IQR) and whiskers extend to 1.5 × IQR in either direction. p-values were calculated via two-sided bootstrapping as detailed in Methods.
Extended Data Fig. 8 Changes in bead radii as a function of temperature for bead codes containing different SAc concentrations.
(a) Measured radii as a function of time for thermo-responsive beads per code upon changing the temperature to 37 °C at time t = 0 s and to 34 °C at time t = 60 s; all traces are offset such that initial bead radii are set to the mean radius for each code at time t = 0 s. The red portion of each trace indicates bead heating from RT to 37 °C; the blue portion of each trace indicates bead cooling from 37 °C to 34 °C. (b) Mean (marker) and standard deviation (error bar) for change in radius upon cooling for beads from each code. n = 5-9 beads for each code; the full dataset is available for download in the OSF repository.
Extended Data Fig. 9 Force multiplex for two TCR55 replicates.
(a) Measured integrated Ca2+ fluorescence signals for each positive cell (light grey markers) and the mean integrated Ca2+ signal (light grey square) for two TCR55 replicates interacting with 4 different peptides at three force ramps for Replicate 1 (top) and Replicate 2 (bottom). The box width defines the interquartile range (IQR) and whiskers extend to 1.5 × IQR in either direction. n = 1498 cells and 1256 cells for rep. #1 and rep. #2, respectively. (b) Correlation plot of the means of integrated Ca2+ signals between two replicates. Dashed black line indicates the line with a slope equal to 1; red dashed line indicates a linear regression. Error bars indicate SEM. (c) Integrated Ca2+ signal as a function of applied force for 4 peptides for Replicate 2. Zero force data is from a TCR55 control experiment where the temperature was maintained at 34 °C; error bars indicate SEM. n = 1256 cells for force ramps experiments and 1320 cells at zero force ramp. Error bars indicate SEM.
Extended Data Fig. 10 Sequence and concentration multiplexed measurements for two TCR55 replicates.
(a) Measured integrated Ca2+ fluorescence signals for each positive cell (light grey markers) and the mean integrated Ca2+ signal (light grey square) for two TCR55 replicates interacting with 4 different peptides at three pMHC concentrations. The box width defines the interquartile range (IQR) and whiskers extend to 1.5 × IQR in either direction. n = 2535 cells and 1699 cells for rep. #1 and rep. #2, respectively. (b) Top row: mean integrated Ca2+ signals for positive cells interacting with VPLTEDALL, VPITEDSQL, VPLTEDAEL and IPLTEEAEL peptides at 1X, 7X and 27X pMHC concentrations and the summarized integrated Ca2+ signals for four selected peptides under three pMHC concentrations for replicate 2. Error bars indicate SEM. (c) Estimated p-value (calculated via two-sided bootstrapping, see Methods) vs. log2-transformed mean fold change of integrated Ca2+ signals for 4 peptides at 3 different pMHC concentrations; grey area represents p-value>0.013 (Bonferroni-corrected p-value at a significance of 0.05). Correspondence between thermo-responsive beads spectral code and displayed peptide sequence combined its concentration is shown at right. (d) Correlation plot of the means of integrated Ca2+ signals between two replicates. Dashed black line indicates the line with a slope equal to 1; red dashed line indicates a linear regression. Error bars indicate SEM.
Supplementary information
Supplementary Information
Supplementary Methods, Discussion, Figs. 1–24 and Tables 1–7.
Supplementary Video 1
Parallel flow focuser for production of thermo-responsive beads with linear amplification (four flow-focusing channels).
Supplementary Video 2
Thermo-response of the thermo-responsive beads with 55 mM sodium acrylate during the heating and cooling process. Code 9 was used in this video.
Supplementary Video 3
Initial bead loading followed by cell loading before on-chip imaging.
Supplementary Video 4
T cells remained in contact with bead surfaces throughout the heating and cooling process. Four representative wells are presented.
Supplementary Video 5
Representative Ca2+ flux within single TCR589 T cells interacting with pMHC-coated thermo-responsive beads bearing the stimulatory HIV-pol peptide (IPLTEEAEL) and the nonstimulatory peptide (VPLTEDAEL).
Supplementary Video 6
Thermo-response of the thermo-responsive beads with 50 mM (code 1), 55 mM (code 15) and 60 mM (code 9) sodium acrylate during the heating and cooling process.
Source data
Source Data Fig. 2
Thermo-responsive beads can display 21 different peptide sequences at physiological densities.
Source Data Fig. 3
Activation responses for TCR589-transduced T cells interacting with a 21-peptide library.
Source Data Fig. 4
Activation responses for TCR55-transduced T cells interacting with 21-peptide library.
Source Data Fig. 5
Multiplexing sequence, applied ramping force and displayed pMHC densities using BATTLES.
Source Data Fig. 6
Activation responses for the clinically tested DMF5 TCR system interacting with 11 peptide sequences from two classes.
Source Data Extended Data Fig. 1
Droplet radius and bead radius.
Source Data Extended Data Fig. 2
Measured storage modulus, loss modulus and modulus of rigidity as a function of angular frequency.
Source Data Extended Data Fig. 3
Calculated coding capacity for thermo-responsive beads.
Source Data Extended Data Fig. 5
Activation responses for TCR589-transduced T cells interacting with a 21-peptide library.
Source Data Extended Data Fig. 6
Measured integrated Ca2+ fluorescence signals for TCR55 negative controls.
Source Data Extended Data Fig. 7
Measured integrated Ca2+ fluorescence signals for TCR55 interacting with PEG-DA beads and thermo-responsive NIPAM beads bearing different stimulatory peptides at different conditions.
Source Data Extended Data Fig. 8
Changes in bead radii as a function of temperature for bead codes containing different SAc concentrations.
Source Data Extended Data Fig. 9
Multiplexing sequence and applied ramping force using BATTLES.
Source Data Extended Data Fig. 10
Multiplexing sequence and displayed pMHC densities using BATTLES.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Y., Zhao, X., White, A.K. et al. A bead-based method for high-throughput mapping of the sequence- and force-dependence of T cell activation. Nat Methods 19, 1295–1305 (2022). https://doi.org/10.1038/s41592-022-01592-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-022-01592-2
This article is cited by
-
Evidence and therapeutic implications of biomechanically regulated immunosurveillance in cancer and other diseases
Nature Nanotechnology (2024)
-
Neoantigens: promising targets for cancer therapy
Signal Transduction and Targeted Therapy (2023)