Abstract
Orexins (also called hypocretins) are hypothalamic neuropeptides that carry out essential functions in the central nervous system; however, little is known about their release and range of action in vivo owing to the limited resolution of current detection technologies. Here we developed a genetically encoded orexin sensor (OxLight1) based on the engineering of circularly permutated green fluorescent protein into the human type-2 orexin receptor. In mice OxLight1 detects optogenetically evoked release of endogenous orexins in vivo with high sensitivity. Photometry recordings of OxLight1 in mice show rapid orexin release associated with spontaneous running behavior, acute stress and sleep-to-wake transitions in different brain areas. Moreover, two-photon imaging of OxLight1 reveals orexin release in layer 2/3 of the mouse somatosensory cortex during emergence from anesthesia. Thus, OxLight1 enables sensitive and direct optical detection of orexin neuropeptides with high spatiotemporal resolution in living animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
DNA and protein sequences of the sensors developed in this study have been deposited on the National Center for Biotechnology Information database (accession nos. MW845970 and MW845971) and are available in the Supplementary Note. The corresponding DNA plasmids for viral production have been deposited both at the VVF (https://vvf.ethz.ch/) and at Addgene. Viral vectors for sensor expression can be obtained either from the Patriarchi laboratory, the VVF or Addgene. All source data are provided with the manuscript. All other raw data can be made available upon reasonable request. Accession codes (Protein Data Bank) are 5WQC, 3SG7, 1WSO and 1CQ0. Source data are provided with this paper.
Code availability
Custom MATLAB and Python code used for data processing and analysis is available at https://github.com/patriarchilab/OxLight1. All other custom code can be made available upon reasonable request.
Change history
30 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41592-022-01449-8
References
Lecea, Lde et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. PNAS 95, 322–327 (1998).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998).
Chemelli, R. M. et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451 (1999).
Hara, J. et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345–354 (2001).
Willie, J. T. et al. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron 38, 715–730 (2003).
Lin, L. et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376 (1999).
Thannickal, T. C. et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 (2000).
Nishino, S., Ripley, B., Overeem, S., Lammers, G. J. & Mignot, E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355, 39–40 (2000).
Peyron, C. et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 6, 991–997 (2000).
Sakurai, T. The role of orexin in motivated behaviours. Nat. Rev. Neurosci. 15, 719–731 (2014).
Peyron, C. et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015 (1998).
Pol, A. Nvanden Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci. 19, 3171–3182 (1999).
Peyron, C. & Kilduff, T. S. Mapping the hypocretin/orexin neuronal system: an unexpectedly productive journey. J. Neurosci. 37, 2268–2272 (2017).
Johnson, P. L. et al. A key role for orexin in panic anxiety. Nat. Med. 16, 111–115 (2010).
Blomeley, C., Garau, C. & Burdakov, D. Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat. Neurosci. 21, 29–32 (2018).
Giardino, W. J. et al. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 21, 1084–1095 (2018).
Mahler, S. V., Moorman, D. E., Smith, R. J., James, M. H. & Aston-Jones, G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci. 17, 1298–1303 (2014).
González, J. A., Iordanidou, P., Strom, M., Adamantidis, A. & Burdakov, D. Awake dynamics and brain-wide direct inputs of hypothalamic MCH and orexin networks. Nat. Commun. 7, 11395 (2016).
Mileykovskiy, B. Y., Kiyashchenko, L. I. & Siegel, J. M. Behavioral correlates of activity in identified hypocretin/orexin. Neurons Neuron 46, 787–798 (2005).
Burdakov, D. et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 50, 711–722 (2006).
Karnani, M. M. et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron 72, 616–629 (2011).
Mahoney, C. E., Cogswell, A., Koralnik, I. J. & Scammell, T. E. The neurobiological basis of narcolepsy. Nat. Rev. Neurosci. 20, 83–93 (2019).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Patriarchi, T. et al. An expanded palette of dopamine sensors for multiplex imaging in vivo. Nat. Methods https://doi.org/10.1038/s41592-020-0936-3 (2020).
Feng, J. et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 102, 745–761 (2019).
Sun, F. et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166 (2020).
Wan, J. et al. A genetically encoded sensor for measuring serotonin dynamics. Nat. Neurosci. https://doi.org/10.1038/s41593-021-00823-7 (2021).
Jing, M. et al. An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat. Methods 17, 1139–1146 (2020).
Ammoun, S. et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Ther. 305, 507–514 (2003).
Hong, C. et al. Structures of active-state orexin receptor 2 rationalize peptide and small-molecule agonist recognition and receptor activation. Nat. Commun. 12, 815 (2021).
Baimel, C., Lau, B. K., Qiao, M. & Borgland, S. L. Projection-target-defined effects of orexin and dynorphin on VTA dopamine neurons. Cell Rep. 18, 1346–1355 (2017).
Muschamp, J. W. et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl Acad. Sci. USA 111, E1648–E1655 (2014).
Smart, D. et al. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 128, 1–3 (1999).
Wan, Q. et al. Mini-G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J. Biol. Chem. 293, 7466–7473 (2018).
Karnani, M. M. et al. Role of spontaneous and sensory orexin network dynamics in rapid locomotion initiation. Prog. Neurobiol. 187, 101771 (2020).
Harris, G. C., Wimmer, M. & Aston-Jones, G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559 (2005).
Gehrlach, D. A. et al. Aversive state processing in the posterior insular cortex. Nat. Neurosci. 22, 1424–1437 (2019).
Sakurai, T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat. Rev. Neurosci. 8, 171–181 (2007).
Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 (2008).
Marcus, J. N. et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 435, 6–25 (2001).
Wenger Combremont, A.-L., Bayer, L., Dupré, A., Mühlethaler, M. & Serafin, M. Slow bursting neurons of mouse cortical layer 6b are depolarized by hypocretin/orexin and major transmitters of arousal. Front. Neurol. https://doi.org/10.3389/fneur.2016.00088 (2016).
Takai, T. et al. Orexin-A is composed of a highly conserved C-terminal and a specific, hydrophilic N-terminal region, revealing the structural basis of specific recognition by the orexin-1 receptor. J. Pept. Sci. 12, 443–454 (2006).
Lee, J.-H. et al. Solution structure of a new hypothalamic neuropeptide, human hypocretin-2/orexin-B. Eur. J. Biochem. 266, 831–839 (1999).
Sievers, F. & Higgins, D. G. Clustal omega, accurate alignment of very large numbers of sequences. Methods Mol. Biol. 1079, 105–116 (2014).
Quan, J. & Tian, J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS ONE 4, e6441 (2009).
Früh, S., Tyagarajan, S. K., Campbell, B., Bosshard, G. & Fritschy, J.-M. The catalytic function of the gephyrin-binding protein IQSEC3 regulates neurotransmitter-specific matching of pre- and post-synaptic structures in primary hippocampal cultures. J. Neurochem. 147, 477–494 (2018).
Mayrhofer, J. M. et al. Design and performance of an ultra-flexible two-photon microscope for in vivo research. Biomed. Opt. Express 6, 4228–4237 (2015).
Xu, C. & Webb, W. W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 13, 481–491 (1996).
Reguardati, S., de, Pahapill, J., Mikhailov, A., Stepanenko, Y. & Rebane, A. High-accuracy reference standards for two-photon absorption in the 680–1050 nm wavelength range. Opt. Express 24, 9053–9066 (2016).
Barnett, L. M., Hughes, T. E. & Drobizhev, M. Deciphering the molecular mechanism responsible for GCaMP6m’s Ca2+-dependent change in fluorescence. PLoS ONE 12, e0170934 (2017).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. eLife 5, e12727 (2016).
Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. Preprint at bioRxiv https://doi.org/10.1101/061507 (2017).
Acknowledgements
The results are part of a project that has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 891959; to T.P.). We also acknowledge funding from the University of Zurich and the Swiss National Science Foundation (grant no. 310030_196455; to T.P.); Forschungskredit Candoc (to X.Z.); ETH Zurich (to D.B.); Swiss National Science Foundation (grant no. PCEFP3_181282; to M.S.); ERC-2017-STG (grant agreement no. 758448; to N.G.); Swiss National Science Foundation and ERC-2016-CoG (grant agreement no. 725850) (A.R.A.); University of Bern and Inselspital University Hospital (A.R.A. and M.H.S.); ERC-2014-CoG (grant agreement no. 647725) and National Institutes of Health Brain Initiative (U19 NS107464) (both to T.F.); H2020-ICT (grant agreement no. 101016787; to T.P. and T.F.). We thank J.-C. Paterna and the VVF of the Neuroscience Center Zurich for help with virus production, M. Drobizhev (Montana State University) for sharing the Rh6G reference two-photon spectrum and useful insights and F. Succol for technical support and for performing IHC and confocal image acquisition. The plasmids coding for mini-Gi-mRuby2, mini-Gs-mRuby2 and mini-Gq-mRuby2 were a kind gift from N. Lambert (Augusta University). The Alexa-647 labeled M1 anti-FLAG antibody was a kind gift from M. von Zastrow (University of California San Francisco).
Author information
Authors and Affiliations
Contributions
T.P. conceived the project and led the study. L.D. performed molecular cloning, in vitro sensor screening and characterization in HEK293 cells and neurons and analyzed data under the supervision of T.P. X.Z. generated the structural model of the sensor and analyzed sensor kinetics under the supervision of T.P. J.D. and L.R. performed the one- and two-photon spectroscopic characterization under the supervision of B.W. and T.P. Y.-C.T. and M.F. prepared hippocampal neuronal cultures under the supervision of S.K.T. A.R.-M. performed TIRF microscopy recruitment assays for mini-G proteins and β-arrestin-2 under the supervision of M.S. S.K. performed viral injections, slice electrophysiology and imaging experiments, IHC and data analysis under the supervision of D.B. E.B. performed surgeries, wrote custom software and performed and analyzed photometry imaging during optogenetic stimulation and running behavior. A.D.Z. performed and analyzed photometry imaging during tail-picking experiments under the supervision of N.G. B.V. performed viral injections, acquired and analyzed EEG, EMG and photometry data during sleep–wake cycles under the supervision of M.H.S. and A.R.A. M.P. performed surgeries and acquired and analyzed in vivo two-photon imaging data under the supervision of T.F. T.P. and L.D. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
T.P. is a co-inventor on a patent application related to the technology described in this article. All other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Yang Dan, Takeharu Nagai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Nina Vogt was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Optimization of cpGFP insertion site into OX2R.
a, Sequence alignment of transmembrane domains (TM) 5 and 6 from OX2R, OX1R and DRD1 indicating the insertion site for the cpGFP module from dLight1. Color code is based on percent identity between orexin receptors and DRD1. b, Representative images showing membrane expression profile of sensor prototypes based on either the OX1 or OX2 receptor in HEK293T cells. c, Membrane expression and fluorescence response to 10 µM OXA of the OX1 and OX2 sensor prototypes shown in a-b. p = 0.3510, for the membrane expression and p = 1.109 × 10−7 for the fluorescence response. n = 15 cells from 3 independent experiments. d-i, cpGFP Insertion point optimization on the TM6 and TM5 of OX2R. The variant showing best membrane expression and response to 10 µM OXA was called OxLight0.1. d, Schematic representation of TM6 insertion site optimization. e, Representative images of TM6 insertion sites variants. f, Quantification of fluorescence responses to 10 µM OXA and surface expression from variants shown in e. p = 0.0047 for OX2 prototype response compared to OxLight0.1. n = 12 and 16 cells for OX2 prototype and OxLight0.1 or ≥ 5 cells for all other variants. g, Schematic representation of TM5 insertion site optimization. h, Representative images of TM5 insertion sites variants. i, Quantification of responses to 10 µM OXA and surface expression from variants shown in h for all variants. j-k, In vitro characterization of OxLight0.1 in HEK293T cells. j, Representative images of OxLight0.1 pre and post addition of 10 µM OXA. k, Response of OxLight0.1 to sequential addition of OXA or OXB followed by dual orexin receptor antagonist EMPA. n = 26 and 31 cells for OXA + EMPA and OXB + EMPA, respectively. All data are shown as mean ± s.e.m. All scale bars, 10 µm. All statistical tests performed using two-tailed student’s t-test with Welch’s correction.
Extended Data Fig. 2 Optimization of OxLight0.1 dynamic range.
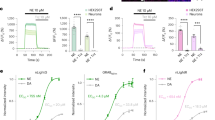
a, Fluorescence responses to 10 µM OXA of intracellular loop 2 (ICL2) variants of OxLight0.1. Left, every amino acid from the ICL2 was individually mutated to an alanine, lysine and glutamate. Right, site-saturated mutagenesis was performed on residue L160 which corresponds to F129 in dLight1. From this screening we selected two mutations (M161K and L160H) each of whom showed significantly higher responses compared to OxLight0.1 (n = 29 cells) (∆F/F0 = 153 ± 8%; p = 3.704 × 10−5 (n = 15 cells); ∆F/F0 = 168 ± 6%; p = 9.954 × 10−6 (n = 14 cells)), respectively). b, Fluorescence responses of OxLight0.1 TM5 variants generated as shown in the inset. The Q254E mutant showed a significantly higher response compared to OxLight0.1 (∆F/F0 = 194 ± 7%; p = 1.349 × 10−8) n = 28 and 20 cells from 3 independent experiments for OxLight0.1 and OxLight0.1-Q to E (TM5) respectively. c, Combination of beneficial mutations from both the ICL2 and TM5 screenings. The triple mutant containing M161K, L160H and Q254E had a greatly improved response: ∆F/F0 = 386 ± 25%; p = 4.843 × 10−9 (n = 19 cells) and was called OxLight0.2. d, Fluorescence responses of OxLight0.2 TM6 variants generated as shown in the inset. The K294R mutant showed a significantly higher response compared to OxLight0.2 (∆F/F0 = 915 ± 14%; p = 1.075 × 10−13 (n = 17 cells) and was called OxLight1. n.s., not significant. All data are shown as mean ± SEM with n ≥ 5 cells. All statistical tests have been performed using a Two-Tailed Student’s t-test with Welch’s correction with data resulting from three independent experiments. *** p < 0.0001.
Extended Data Fig. 3 Development and characterization of a control sensor.
a, Representative images of HEK cells expressing different mutants of OxLight1, indicated by absolute residue numbering from the N-terminus of OX2R. b, Quantification of maximal ∆F/F0 in response to bath-applied orexin-A and orexin-B (both at 10 µM) for all mutants in a. The OxLight1 E54K + T111A mutant has a significantly decreased response compared to OxLight1: p = 2.174 × 10−21 with n = 32 and 25 cells, respectively. c, The two OxLight1 sites selected for the control sensor are highlighted in magenta in the structural model of OxLight1. Residue sidechains are shown for the two amino acid positions E541.32 and T1112.61. d, Representative images of OxLight-ctr expression in HEK cells and ∆F/F0 after addition of OXA or OXB. e, Identical to d, but in primary cultured neurons. f, Left, membrane ∆F/F0 in OxLight1 or OxLight-ctr expressing HEK cells from time lapse imaging experiments. Bars indicate bath application of orexins (black, both at 10 µM) followed by the OX2R antagonist EMPA (magenta, 10 µM). Right, quantification of maximal ∆F/F0 from time lapse imaging experiments shown on left. n = 16 and 21, cells for OxLight1 OXA HEK compared to OxLight-ctr OXA HEK (p = 1.640 × 10−19). n = 10 and 22 cells for OxLight1 OXB HEK compared to OxLight-ctr OXB HEK (p = 5.926 × 10−11), Data are shown as mean ± SEM and all statistical analysis performed using Two tailed student’s t-test with Welch’s correction from 3 independent experiments. *** p < 0.0001. All scale bars, 10 µm.
Extended Data Fig. 4 Spectral properties of OxLight1.
a, Left, One-photon fluorescence excitation (λ emission = 560 nm) and emission (λ excitation = 470 nm) spectra acquired from OxLight1-expressing HEK cells in the absence (No OXA) or presence (OXA) of orexin-A (OXA, 10 µM). Each trace is the average of 3 independent experiments (a.u. = arbitrary units). Right, ratio of fluorescence excitation spectra shown on left. b, Left, relative two photon brightness of OxLight1 expressed in HEK cells grown adherent on a glass coverslip. Each trace is the average of 3 independent experiments. Right, ratio of traces shown on left. In both a and b fluorescence excitation and emission were normalized to the respective maximal value in the absence of OXA.
Extended Data Fig. 5 Brightness and p.H. sensitivity of OxLight1.
a, OxLight1 and OxLight-ctr brightness assessment in the presence or absence of 10 µM OXA compared to OX2R with C-terminally tagged GFP in HEK293T cells. All the data were normalized to OX2R-GFP mean fluorescence intensity. Representative images for each condition shown on top right, scale bars, 10 µm. p = 0,6367 for OxLight1+OXA compared to OX2R-GFP, two-tailed student’s t test with Welch’s correction. n = 104 cells from 3 different experiments for each condition. b, Comparison of OxLight1 fluorescence response to an equimolar mix of OXA and OXB (5 µM) (black) in PBS solutions at different pH levels. n = 60; 51; 53; 62; 63; 69; 71; 66; 57; 55; 69; 73; 57; 59; 52; 61; 53; 58; 51; 46; 51 cells for each dataset from p.H. 6 to 8, respectively. Each dataset was obtained from 3 independent experiments. All data are shown as mean ± SEM.
Extended Data Fig. 6 Additional characterization of OxLight1.
a, Characterization of OxLight1 coupling to intracellular calcium activity in primary rat hippocampal neurons. Intracellular calcium dynamics were measured in co-transduced neurons expressing OxLight1 and jRCaMP1b. ΔF/F0 responses of jRCaMP1b were recorded after addition of vehicle (ACSF + 1 µM TTX), followed by 1 nM and 500 nM equimolar mix of OXA and OXB. Ligand addition is indicated by colored bars. Maximal intracellular calcium response was elicited with 10 µM ionomycin as a positive control. n = 41 neurons from 5 different experiments. b, Quantification of responses from a. Individual data points represent the average jRCaMP1b ΔF/F0 response of individual neurons for each condition. One-way ANOVA (p = 1.748 × 10−130) followed by Bonferroni’s multiple comparison test comparing vehicle against 1 nM OXA-OXB (adjusted p = 0,9601), 500 nM OXA-OXB (adjusted p = 0,6052) and 10 µM ionomycin (p = 9.685 × 10−118). ns, non-significant (p > 0.016); *** p < 0.0001 c, Representative images of neurons used in a-b. Scale bar, 10 µm. d, Representative images of OxLight1 fluorescence at the indicated time points before and after addition of OXB (5 µM) and almorexant (10 µM). Scale bar, 10 µm. e, Normalized fluorescence response values for OxLight1-expressing cells as in d. n = 24 cells from 3 different experiments. *** p < 0.0001, n.s. not significant. Brown-Forsythe one way ANOVA test comparing 10, 30, 60 and 90 min: p = 0.1221; Brown-Forsythe one way ANOVA test followed by post hoc Dunnett’s T3 multiple comparison test to compare HBSS to 10, 30, 60, 90 min and Almorexant. p = 0.2042 between HBSS and Almorexant. All data are shown as mean ± SEM.
Extended Data Fig. 7 Characterization of sensor coupling to intracellular signaling partners.
a-e, Mini-G protein recruitment to wild-type orexin type-2 receptor (OX2R) or OxLight1. a, Agonist-induced membrane recruitment of mRuby-tagged mini-Gq, mini-Gs, mini-Gi or mini-G12 probes (collectively mini-Gx) in OX2R-expressing HEK293 cells upon OXB addition (50 nM). TIRF time-lapse movies frame-rate was 0.2/s. Signal quantification: agonist-induced ratio-change of mRuby-fluorescence compared to baseline (∆R/R0). n = 3 independent experiments for each mini-G. b, Membrane-recruitment normalized to M1-Alexa-647 fluorescence (before vs. 5 min after OXB addition) observed for constructs tested in a. Mini-Gq (n = 19 cells) compared to mini-Gi (n = 17 cells, p = 4.541×10−5), mini-Gs (n = 15 cells, p = 7.755×10−6) and miniG12 (n = 11 cells, p = 6.816×10−6). c, Recruitment of mRuby-tagged-mini-Gq measured as in a in OX2R- or OxLight1-expressing HEK293 cells (n = 3 independent experiments). d, Membrane-recruitment normalized to M1-Alexa-647 fluorescence (before vs. 5 minutes after OXB treatment) observed for constructs tested in c. p = 2.196×10−5 for OxLight1 compared to OX2R. n = 19 and 14 cells for OX2R and OxLight1, respectively. e, Membrane-recruitment (before vs. 5 minutes after OXB treatment) of mRuby-tagged-mini-Gs, mini-Gi or mini-G12 monitored in OxLight1-expressing HEK293 cells upon OXB addition (50 nM) compared to baseline (∆R/R0) and normalized to M1-Alexa-647 fluorescence. n = 19, n = 21 and 17 cells (n = 3 independent experiments) for mini-G12, mini-Gi and mini-Gs respectively. f, Membrane-recruitment of mCherry-tagged-beta-Arrestin-2 to activated OX2R or OxLight1 upon OXB addition (50 nM), measured as in a. and normalized to M1-Alexa-647 fluorescence g, Membrane-recruitment of mCherry-tagged-beta-Arrestin-2 (before vs. 15 min after OXB addition) observed for each construct tested in f. p = 4.603×10−6 for OxLight1 compared to OX2R. n = 19 and 23 cells (n = 5 independent experiments for OX2R and OxLight1, respectively). h, Representative images of cells from g at baseline and 15 minutes after stimulation with OXB (50 nM). Scale bars, 10 µm. All data shown as mean ± SEM, two-tailed student’s t test with Welch’s correction for all statistical analyses.
Extended Data Fig. 8 Dynamic orexin fluctuations during brain states.
a, Schematic drawing of AAV injections in the LH and experimental setup for recording EEG, EMG and fiber photometry during sleep–wake states. b, Representative recordings showing (top to bottom) EEG spectrogram, EEG, EMG, hypnogram, and ΔF/F0 fluorescence trace of an OxLight1-injected animal. Left, example of a wake-predominant 30-minute recording, and right, example of a REM-predominant 30-minute recording. Both traces belong to the same OxLight1-injected animal. Hypnogram color-code: wake = green, NREM = blue, REM = orange. c, Medetomidine and isoflurane-induced modulation of OxLight1 signal in OxLight1-injected mice. Left, bar plots graph showing mean ΔF/F ± SEM of the sleep–wake and anesthetic-dependent states (medetomidine top left, isoflurane bottom left). Statistical analysis was performed using one-way ANOVA with Bonferroni’s multiple comparisons test n = 4 mice, p = 0.0191 for NREM vs REM (Medetomidine), p = 0.0216 for REM vs Medetomidine, p = 0.0118 for Wake vs REM, p = 0.0062 for Wake vs Isofuorane, p = 0.0032 for NREM vs REM (isoflurane), p = 0.0017 for REM vs Isofluorane. Right, representative hypnogram and trace of OxLight1 fluorescence in one-hour recording during medetomidine injection (top right) and isoflurane exposure (bottom right). Time spent under isoflurane-induced anesthesia: 12 minutes, time spent in medetomidine-induced anesthesia: 30 minutes.
Extended Data Fig. 9 Immunohistochemical verification of orexinergic fibers in the somatosensory cortex.
a, A coronal section of the somatosensory cortex of one mouse showing the laminar spread of OxLight1. Scale bar, 100 µm. b, A high-magnification image (40x) of one coronal section from a brain expressing OxLight-ctr (green) in the somatosensory cortex layer 2/3 (L2/3). The section was stained with Hoechst dye (blue) and with an anti-Orexin-A antibody (white). Scale bar, 50 µm.
Extended Data Fig. 10 Characterization of OxLight1 and OxLight-ctr expression in somatosensory cortex.
a, Distributions of pixel-intensities for maximum-projections shown in Fig. 6b top-left (dark grey) and bottom left (light grey) from one FOV in a mouse expressing OxLight1. b, Same as in a for OxLight-ctr, related to Fig. 6b top-right (dark grey) and bottom right (light grey). c, Fluorescence traces representing frame-by-frame average fluorescence from all pixels in 2 FOVs from 2 mice expressing OxLight1 (left) or OxLight-ctr (right). Anesthesia was off for the first 2 minutes of imaging. d, Raw-fluorescence traces for 2 example OxLight1-FOVs and 2 example OxLight-ctr-FOVs, corresponding to a time window between the 3rd and 6th minute of imaging in each FOV. Boxes highlight the most-active minute. e, Mean across-frames s.d. calculated during the first minute of imaging and during the most-active minute, for each OxLight1 FOV (light-green) and OxLight-ctr-FOV (dark-green). Solid lines indicate mean across FOVs. OxLight1 data are normally distributed (paired t-test; p = 0.0013), while OxLight-ctr data are not (Wilcoxon signed-rank test; p = 0.57). f, Median pixel intensity values during the anaesthetized period (F0) in 8 OxLight1-FOVs and 8 OxLight-ctr-FOVs. The two distributions are not significantly different (unpaired t-test; p = 0.41). g, Heatmaps showing the frame-by-frame deconvolved activity in 20 ROIs from one example OxLight1-FOV (left) and one example OxLight-ctr-FOV (right). Solid vertical grey lines indicate the most-active minute. FOVs are the same as in Fig. 6 f. h, Heatmaps showing frame-by-frame raw fluorescence of each ROI in 2 example OxLight1-FOVs. Yellow lines indicate the most-active minute. i, Same as in h, but for 2 example OxLight-ctr-FOVs. j, Pearson’s correlation coefficients between all ROI pairs in 2 example OxLight1-FOVs, during the most-active minute. k, Same as in j, but for two example OxLight-ctr-FOVs. l, Pearson’s correlation coefficients in 8 OxLight1-FOVs across all pairs of active and inactive ROIs, during most-active minute (mean + /- SEM) (Wilcoxon signed-rank test; p = 0.0207).
Supplementary information
Supplementary Information
Supplementary Note and Supplementary Figs. 1–3.
Supplementary Video 1
Two-photon movie of an FOV acquired in the somatosensory cortex of a mouse expressing OxLight1 in layer 2/3. Isoflurane anesthesia was on for the first minute of imaging and was then turned off while the mouse was allowed to recover. Scale bar, 50 μm. Images were acquired at 5 Hz.
Supplementary Video 2
Two-photon movie of an FOV acquired in the somatosensory cortex of a mouse expressing OxLight-ctr in layer 2/3. Isoflurane anesthesia was on for the first minute of imaging and was then turned off while the mouse was allowed to recover. Scale bar, 50 μm. Images were acquired at 5 Hz.
Source data
Source Data Fig. 1
Source data from the development of the orexin sensor.
Source Data Fig. 2
Source data from the sensor characterization in vitro.
Source Data Fig. 3
Source data from the ex vivo and in vivo validation of the sensor.
Source Data Fig. 4
Source data from the monitoring orexin dynamics associated with natural behaviors.
Source Data Fig. 5
Source data from the tracking of orexin dynamics across sleep–wake cycles.
Source Data Fig. 6
Source data from the two-photon imaging of cortical orexin dynamics during emergence from anesthesia.
Source Data Extended Data Fig. 1
Source data from the optimization of cpGFP insertion site into OX2R.
Source Data Extended Data Fig. 2
Source data from the optimization of OxLight0.1 dynamic range.
Source Data Extended Data Fig. 3
Source data from the development and characterization of a control sensor.
Source Data Extended Data Fig. 4
Source data from the spectral properties of OxLight1.
Source Data Extended Data Fig. 5
Source data from the brightness and pH sensitivity of OxLight1.
Source Data Extended Data Fig. 6
Source data from the additional characterization of OxLight1.
Source Data Extended Data Fig. 7
Source data from the characterization of sensor coupling to intracellular signaling partners.
Source Data Extended Data Fig. 8
Source data from the dynamic orexin fluctuations during brain states.
Source Data Extended Data Fig. 10
Source data from the characterization of OxLight1 and OxLight-ctr expression in somatosensory cortex.
Rights and permissions
About this article
Cite this article
Duffet, L., Kosar, S., Panniello, M. et al. A genetically encoded sensor for in vivo imaging of orexin neuropeptides. Nat Methods 19, 231–241 (2022). https://doi.org/10.1038/s41592-021-01390-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01390-2
This article is cited by
-
A genetically encoded sensor for visualizing leukotriene B4 gradients in vivo
Nature Communications (2023)
-
Sensitive multicolor indicators for monitoring norepinephrine in vivo
Nature Methods (2023)
-
Control and coding of pupil size by hypothalamic orexin neurons
Nature Neuroscience (2023)