Abstract
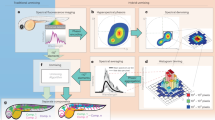
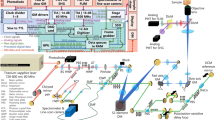
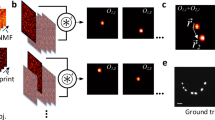
Fluorescence lifetime imaging microscopy (FLIM) and spectral imaging are two broadly applied methods for increasing dimensionality in microscopy. However, their combination is typically inefficient and slow in terms of acquisition and processing. By integrating technological and computational advances, we developed a robust and unbiased spectral FLIM (S-FLIM) system. Our method, Phasor S-FLIM, combines true parallel multichannel digital frequency domain electronics with a multidimensional phasor approach to extract detailed and precise information about the photophysics of fluorescent specimens at optical resolution. To show the flexibility of the Phasor S-FLIM technology and its applications to the biological and biomedical field, we address four common, yet challenging, problems: the blind unmixing of spectral and lifetime signatures from multiple unknown species, the unbiased bleedthrough- and background-free Förster resonance energy transfer analysis of biosensors, the photophysical characterization of environment-sensitive probes in living cells and parallel four-color FLIM imaging in tumor spheroids.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The code for performing blind unmixing is provided, together with a script for simulating S-FLIM data for testing and an example of experimental S-FLIM data. Further code is available from the corresponding author upon reasonable request.
References
Becker, W., Bergmann, A., Biscotti, G. L. & Rueck, A. Advanced time-correlated single photopy and imaging in biomedical systems. In Proc. Commercial and Biomedical Applications of Ultrafast Lasers IV: Lasers and Applications in Science and Engineering Conference (eds. Neev, J. et al.) (SPIE, 2004); https://doi.org/10.1117/12.529143
Owen, D. M. et al. Excitation-resolved hyperspectral fluorescence lifetime imaging using a UV-extended supercontinuum source. Opt. Lett. 32, 3408–3410 (2007).
Fereidouni, F., Reitsma, K. & Gerritsen, H. C. High speed multispectral fluorescence lifetime imaging. Opt. Express 21, 11769–11782 (2013).
Borlinghaus, R. & Kuschel, L. Spectral fluorescence lifetime imaging microscopy: new dimensions with Leica TCS SP5. Nat. Methods 3, 868 (2006).
König, K. (ed.) Multiphoton Microscopy and Fluorescence Lifetime Imaging: Applications in Biology and Medicine (De Gruyter, 2018); https://doi.org/10.1515/9783110429985
Yan, L., Rueden, C. T., White, J. G. & Eliceiri, K. W. Applications of combined spectral lifetime microscopy for biology. BioTechniques 41, 249, 251, 253 (2006).
Rueck, A. C., Lorenz, S., Hauser, C., Mosch, S. & Kalinina, S. Multiwavelength FLIM: new concept for fluorescence diagnosis. In Proc. Multiphoton Microscopy in the Biomedical Sciences XII (ed. König, K.) (SPIE, 2012); https://doi.org/10.1117/12.906620
Levenson, R. M., Lynch, D. T., Kobayashi, H., Backer, J. M. & Backer, M. V. Multiplexing with multispectral imaging: from mice to microscopy. ILAR J. 49, 78–88 (2008).
Gratton, E. Fluorescence lifetime imaging for the two-photon microscope: time-domain and frequency-domain methods. J. Biomed. Opt. https://doi.org/10.1117/1.1586704 (2003).
Jameson, D. M., Gratton, E. & Hall, R. D. The measurement and analysis of heterogeneous emissions by multifrequency phase and modulation fluorometry. Appl. Spectrosc. Rev. https://doi.org/10.1080/05704928408081716 (1984).
Lou, J. et al. Phasor histone FLIM-FRET microscopy quantifies spatiotemporal rearrangement of chromatin architecture during the DNA damage response. Proc. Natl Acad. Sci. USA 116, 7323–7332 (2019).
Digman, M. A., Caiolfa, V. R., Zamai, M. & Gratton, E. The phasor approach to fluorescence lifetime imaging analysis. Biophys. J. 94, 14–16 (2008).
Scipioni, L., Gratton, E., Diaspro, A. & Lanzanò, L. Phasor analysis of local ICS detects heterogeneity in size and number of intracellular vesicles. Biophys. J. 111, 619S (2016).
Ranjit, S., Malacrida, L., Jameson, D. M. & Gratton, E. Fit-free analysis of fluorescence lifetime imaging data using the phasor approach. Nat. Protoc. 13, 1979–2004 (2018).
Sarmento, M. J. et al. Exploiting the tunability of stimulated emission depletion microscopy for super-resolution imaging of nuclear structures. Nat. Commun. 9, 3415 (2018).
Hanley, Q. S. Spectrally resolved fluorescent lifetime imaging. J. R. Soc. Interface 6, S83–S92 (2009).
Cutrale, F., Salih, A. & Gratton, E. Spectral phasor approach for fingerprinting of photo-activatable fluorescent proteins Dronpa, Kaede and KikGR. Methods Appl. Fluoresc. 1, 35001 (2013).
Cutrale, F. et al. Hyperspectral phasor analysis enables multiplexed 5D in vivo imaging. Nat. Methods 14, 149–152 (2017).
Fereidouni, F., Bader, A. N. & Gerritsen, H. C. Spectral phasor analysis allows rapid and reliable unmixing of fluorescence microscopy spectral images. Opt. Express 20, 12729–12741 (2012).
Ranjit, S., Malacrida, L. & Gratton, E. Differences between FLIM phasor analyses for data collected with the Becker and Hickl SPC830 card and with the FLIMbox card. Microsc. Res. Tech. 81, 980–989 (2018).
Ma, W. K. et al. A signal processing perspective on hyperspectral unmixing: Insights from remote sensing. IEEE Signal Process Mag. https://doi.org/10.1109/MSP.2013.2279731 (2014).
Keshava, N. A survey of spectral unmixing algorithms. Linc. Lab. J. 14, 55–78 (2003).
McRae, T. D., Oleksyn, D., Miller, J. & Gao, Y. R. Robust blind spectral unmixing for fluorescence microscopy using unsupervised learning. PLoS ONE 14, e0225410 (2019).
Neher, R. A. et al. Blind source separation techniques for the decomposition of multiply labeled fluorescence images. Biophys. J. 96, 3791–3800 (2009).
Medintz, I. & Hildebrandt, N. (eds.) FRET—Förster Resonance Energy Transfer: From Theory to Applications (Wiley, 2013); https://doi.org/10.1002/9783527656028
Gopich, I. V. & Szabo, A. Theory of the energy transfer efficiency and fluorescence lifetime distribution in single-molecule FRET. Proc. Natl Acad. Sci. USA 109, 7747–7752 (2012).
Vallmitjana, A., Torrado, B., Dvornikov, A., Ranjit, S. & Gratton, E. Blind resolution of lifetime components in individual pixels of fluorescence lifetime images using the phasor approach. J. Phys. Chem. B 124, 10126–10137 (2020).
Koushik, S. V., Blank, P. S. & Vogel, S. S. Anomalous surplus energy transfer observed with multiple FRET acceptors. PLoS ONE 4, e8031 (2009).
Koushik, S. V., Chen, H., Thaler, C., Puhl, H. L. & Vogel, S. S. Cerulean, venus, and venusY67C FRET reference standards. Biophys. J. 91, L99–L101 (2006).
Peng, Q. et al. Coordinated histone modifications and chromatin reorganization in a single cell revealed by FRET biosensors. Proc. Natl Acad. Sci. USA 115, E11681–E11690 (2018).
Pelicci, S., Diaspro, A. & Lanzanò, L. Chromatin nanoscale compaction in live cells visualized by acceptor-to-donor ratio corrected Förster resonance energy transfer between DNA dyes. J. Biophotonics 12, e201900164 (2019).
Algar, W. R., Hildebrandt, N., Vogel, S. S. & Medintz, I. L. FRET as a biomolecular research tool—understanding its potential while avoiding pitfalls. Nat. Methods 16, 815–829 (2019).
Hellenkamp, B. et al. Precision and accuracy of single-molecule FRET measurements—a multi-laboratory benchmark study. Nat. Methods 15, 669–676 (2018).
Bagatolli, L. A. in Fluorescent Methods to Study Biological Membranes (eds. Mély, Y. & Duportail, G.) 3–35 (Springer, 2012).
Weber, G. & Farris, F. J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry 18, 3075–3078 (1979).
Parasassi, T. & Gratton, E. Membrane lipid domains and dynamics as detected by Laurdan fluorescence. J. Fluoresc. 5, 59–69 (1995).
Parasassi, T., Krasnowska, E. K., Bagatolli, L. & Gratton, E. Laurdan and prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 8, 365–373 (1998).
Swain, J. & Mishra, A. K. Nile red fluorescence for quantitative monitoring of micropolarity and microviscosity of pluronic F127 in aqueous media. Photochem. Photobiol. Sci. 15, 1400–1407 (2016).
Kreder, R. et al. Solvatochromic Nile Red probes with FRET quencher reveal lipid order heterogeneity in living and apoptotic cells. ACS Chem. Biol. 10, 1435–1442 (2015).
Sanchez, S. A., Tricerri, M. A., Gunther, G. & Gratton, E. in Modern Research and Educational Topics in Microscopy (eds. Méndez-Vilas, A. & Díaz, J.) 1007–1014 (FORMATEX, 2007).
Sanchez, S. A., Tricerri, M. A. & Gratton, E. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc. Natl Acad. Sci. USA 109, 7314–7319 (2012).
Bückers, J., Wildanger, D., Vicidomini, G., Kastrup, L. & Hell, S. W. Simultaneous multi-lifetime multi-color STED imaging for colocalization analyses. Opt. Express 19, 3130–3143 (2011).
Laviv, T. et al. Simultaneous dual-color fluorescence lifetime imaging with novel red-shifted fluorescent proteins. Nat. Methods 13, 989–992 (2016).
Yamada, K. M. & Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 (2007).
Tevis, K. M., Colson, Y. L. & Grinstaff, M. W. Embedded spheroids as models of the cancer microenvironment. Adv. Biosyst. https://doi.org/10.1002/adbi.201700083 (2017).
Ramanujan, V. K. Quantitative imaging of morphometric and metabolic signatures reveals heterogeneity in drug response of three-dimensional mammary tumor spheroids. Mol. Imaging Biol. 21, 436–446 (2019).
Avagliano, A. et al. Mitochondrial flexibility of breast cancers: a growth advantage and a therapeutic opportunity. Cells 8, 401 (2019).
Smyrek, I. et al. E-cadherin, actin, microtubules and FAK dominate different spheroid formation phases and important elements of tissue integrity. Biol. Open 8, bio037051 (2019).
Vidavsky, N. et al. Mapping and profiling lipid distribution in a 3D model of breast cancer progression. ACS Cent. Sci. 5, 768–780 (2019).
Sivandzade, F., Bhalerao, A. & Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc. 9, e3128 (2019).
Vallmitjana, A. et al. Resolution of 4 components in the same pixel in FLIM images using the phasor approach. Methods Appl. Fluoresc. 8, 035001 (2020).
Navarro-Tito, N., Soto-Guzman, A., Castro-Sanchez, L., Martinez-Orozco, R. & Salazar, E. P. Oleic acid promotes migration on MDA-MB-231 breast cancer cells through an arachidonic acid-dependent pathway. Int. J. Biochem. Cell Biol. 42, 306–317 (2010).
Abramczyk, H. et al. The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7, and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst 140, 2224–2235 (2015).
Heerdt, B. G., Houston, M. A. & Augenlicht, L. H. The intrinsic mitochondrial membrane potential of colonic carcinoma cells is linked to the probability of tumor progression. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-05-2444 (2005).
Reynolds, D. S. et al. Breast cancer spheroids reveal a differential cancer stem cell response to chemotherapeutic treatment. Sci. Rep. 7, 10382 (2017).
Szlasa, W., Zendran, I., Zalesińska, A., Tarek, M. & Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 52, 321–342 (2020).
Kremers, G. J., Goedhart, J., Van Munster, E. B. & Gadella, T. W. J. Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET förster radius. Biochemistry 45, 6570–6580 (2006).
Goedhart, J. et al. Bright cyan fluorescent protein variants identified by fluorescence lifetime screening. Nat. Methods 7, 137–139 (2010).
Rizzo, M. A., Springer, G. H., Granada, B. & Piston, D. W. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22, 445–449 (2004).
Acknowledgements
We thank F. Palomba, A. Vallmitjana Lees, C. Gohlke and A. Dvornikov for the useful discussion and D. Jameson for his input on the paper. This work was supported by grant no. NIH P41-GM103540.
Author information
Authors and Affiliations
Contributions
L.S. and E.G. conceived the idea. A.R. developed and wrote FPGA code. L.S., A.R. and E.G. wrote code. L.S. designed electronics, built the microscope and performed simulations and experiments. G.T. prepared all biological samples. L.S. wrote the paper with input from all authors
Corresponding author
Ethics declarations
Competing interests
A.R. declares his involvement in FLIM LABS Srl, Rome, Italy.
Additional information
Peer review information Nature Methods thanks Luis Alvarez, Mantas Zurauskas and Francesco Cutrale for their contribution to the peer review of this work. Rita Strack was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, information on various technical aspects, Tables 1 and 2 and bibliography.
Supplementary Software
File contains example of S-FLIM dataset (SFLIM_Dataset.mat), calibration file (20200319.mat) and script to run the unmixing (SFLIM_Unmixing_Solution.mat).
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
About this article
Cite this article
Scipioni, L., Rossetta, A., Tedeschi, G. et al. Phasor S-FLIM: a new paradigm for fast and robust spectral fluorescence lifetime imaging. Nat Methods 18, 542–550 (2021). https://doi.org/10.1038/s41592-021-01108-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01108-4
This article is cited by
-
More than double the fun with two-photon excitation microscopy
Communications Biology (2024)
-
Live-cell imaging powered by computation
Nature Reviews Molecular Cell Biology (2024)
-
HyU: Hybrid Unmixing for longitudinal in vivo imaging of low signal-to-noise fluorescence
Nature Methods (2023)
-
Multicolor lifetime imaging and its application to HIV-1 uptake
Nature Communications (2023)
-
Engineered HaloTag variants for fluorescence lifetime multiplexing
Nature Methods (2022)