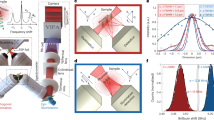
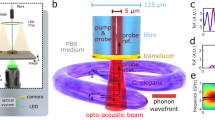
Abstract
Label-free, non-contact imaging with mechanical contrast and optical sectioning is a substantial challenge in microscopy. Spontaneous Brillouin scattering microscopy meets this challenge, but encounters a trade-off between acquisition speed and the specificity for biomechanical constituents with overlapping Brillouin bands. Stimulated Brillouin scattering microscopy overcomes this trade-off and enables the cross-sectional imaging of live Caenorhabditis elegans at the organ and subcellular levels, with both elasticity and viscosity contrasts at high specificity and with practical recording times.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All imaging and spectral data that support the findings of this study and which are not available from public repositories owing to university constraints are available from the corresponding authors upon reasonable request and with the permission of Ben-Gurion University of the Negev.
Code availability
The SBS image reconstruction codes that support the findings of this study are available from the corresponding authors upon reasonable request and with the permission of Ben-Gurion University of the Negev under the BSD license. The code used to analyze the spectral data is provided under the BSD license as Supplementary Software along with representative sample data. The LabVIEW program for controlling the microscope, which is hardware-dependent, is available from the corresponding author upon request.
Change history
20 August 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41592-020-0956-z
References
Thomas, G., Burnham, N. A., Camesano, T. A. & Wen, Q. Measuring the mechanical properties of living cells using atomic force microscopy. J. Vis. Exp. 76, 50497 (2013).
Kennedy, B. F., Wijesinghe, P. & Sampson, D. D. The emergence of optical elastography in biomedicine. Nat. Photonics 11, 215–221 (2017).
Chen, X., Nadiarynkh, O., Plotnikov, S. & Campagnola, P. J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 8, 654–669 (2012).
Palombo, F., Madami, M., Stoneac, N. & Fioretto, D. Mechanical mapping with chemical specificity by confocal Brillouin and Raman microscopy. Analyst 139, 729–733 (2014).
Scarcelli, G. et al. Noncontact three-dimensional mapping of intracellular hydromechanical properties by Brillouin microscopy. Nat. Methods 12, 1132–1134 (2015).
Antonacci, G. et al. Quantification of plaque stiffness by Brillouin microscopy in experimental thin cap fibroatheroma. J. R. Soc. Interface 12, 20150483 (2015).
Elsayad, K. et al. Mapping the subcellular mechanical properties of live cells in tissues with fluorescence emission-Brillouin imaging. Sci. Signal 9, rs5 (2016).
Scarponi, F. et al. High-performance versatile setup for simultaneous Brillouin-Raman microspectroscopy. Phys. Rev. X 7, 031015 (2017).
Mattana, S. et al. Non-contact mechanical and chemical analysis of single living cells by microspectroscopic techniques. Light Sci. Appl. 7, 17139 (2018).
Akilbekova, D. et al. Brillouin spectroscopy and radiography for assessment of viscoelastic and regenerative properties of mammalian bones. J. Biomed. Opt. 23, 1–11 (2018).
Schlußler, R. et al. Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by Brillouin imaging. Biophys. J. 115, 911–923 (2018).
Andriotis, O. G. et al. Hydration and nanomechanical changes in collagen fibrils bearing advanced glycation end-products. Biomed. Opt. Express 10, 1841–1855 (2019).
Nikolić, M. & Scarcelli, G. Long-term Brillouin imaging of live cells with reduced absorption-mediated damage at 660nm wavelength. Biomed. Opt. Express 10, 1567–1580 (2019).
Zhang, J. et al. Tissue biomechanics during cranial neural tube closure measured by Brillouin microscopy and optical coherence tomography. Birth Defects Res. 111, 991–998 (2019).
Prevedel, R., Diz-Muñoz, A., Ruocco, G. & Antonacci, G. Brillouin microscopy: an emerging tool for mechanobiology. Nat. Methods 16, 969–977 (2019).
Boyd, R. W. Nonlinear Optics (Academic Press, 2008).
Remer, I. & Bilenca, A. Background-free Brillouin spectroscopy in scattering media at 780 nm via stimulated Brillouin scattering. Opt. Lett. 41, 926–929 (2016).
Remer, I. & Bilenca, A. High-speed stimulated Brillouin scattering spectroscopy at 780 nm. APL Photonics 1, 061301 (2016).
Vaughan, J. M. & Randall, J. T. Brillouin scattering, density and elastic properties of the lens and cornea of the eye. Nature 284, 489–491 (1980).
Ballmann, C. W. et al. Stimulated Brillouin scattering microscopic imaging. Sci. Rep. 5, 18139 (2015).
Ballmann, C. W., Meng, Z., Traverso, A. J., Scully, M. O. & Yakovlev, V. V. Impulsive Brillouin microscopy. Optica 4, 124–128 (2017).
Krug, B., Koukourakis, N. & Czarske, J. W. Impulsive stimulated Brillouin microscopy for non-contact, fast mechanical investigations of hydrogels. Opt. Express 27, 26910–26923 (2019).
Park, S. J., Goodman, M. B. & Pruitt, B. L. Analysis of nematode mechanics by piezoresistive displacement clam. Proc. Natl Acad. Sci. USA 104, 17376–17381 (2007).
Choi, W. et al. Tomographic phase microscopy. Nat. Methods 4, 717–719 (2007).
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Hu, C. R. et al. Stimulated Raman scattering imaging by continuous-wave laser excitation. Opt. Lett. 38, 1479–1481 (2013).
Zhang, J., Fiore, A., Yun, S. H., Kim, H. & Scarcelli, G. Line-scanning Brillouin microscopy for rapid non-invasive mechanical imaging. Sci. Rep. 6, 35398 (2016).
Remer, I., Cohen, L. & Bilenca, A. High-speed continuous-wave stimulated Brillouin scattering spectrometer for material analysis. J. Vis. Exp. 127, e55527 (2017).
Polyanskiy, M. N. Refractive Index Database (2008–2019); http://www.refractiveindex.info
Podeǎ, J., Procházka, O. & Medin, A. Studies on agaroses; 1. Specific refractive index increments in dimethyl sulfoxide and in water at various wavelengths and temperatures. Polymer 36, 4967–4970 (1995).
Weast, R. C. Handbook of Chemistry and Physics (CRC Press, 1987).
Acknowledgements
A.B. acknowledges the support of the Israel Science Foundation (grant no. 1173/17). I.R. acknowledges the support of the Azrieli Foundation.
Author information
Authors and Affiliations
Contributions
I.R. and A.B. initiated and supervised the project; N.S and A.B.-Z. developed the C. elegans protocol. I.R., R.S. and N.S. conducted the experiments; I.R. and A.B. developed the SBS microscope and analyzed the data; I.R. and A.B. wrote the manuscript and all the coauthors contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
I.R. is an employee of Agilent Technologies. All other authors declare no competing financial interests.
Additional information
Peer review information Nina Vogt was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–7, Tables 1 and 2, Notes 1–4 and References
Supplementary Video 1
Cross-sectional SBS imaging of the head of the same nematode presented in Fig. 1e and under the same conditions. At each image pixel, the measured SBG spectrum was fitted to a double-Lorentzian line shape to retrieve the SBG spectral parameters of the mechanical constituent of the nematode. Seventeen z-stacks were acquired with 5-μm intervals and 0.25-NA lenses. At depths closer to the agar pads, the SBG spectrum measured in the tissue that surrounds the pharynx includes a dominant SBG component of the agar (Supplementary Note 4). This limits the accuracy with which the SBG spectral parameters of this tissue was retrieved and results in variations with depth of these parameters. Images contain 100 × 200 pixels, resulting in total image and video recording times of 400 s and 113 min, respectively. Scale bar, 20 μm.
Supplementary Software
Matlab code that analyzes spectral data along with representative Supplementary Data.
Rights and permissions
About this article
Cite this article
Remer, I., Shaashoua, R., Shemesh, N. et al. High-sensitivity and high-specificity biomechanical imaging by stimulated Brillouin scattering microscopy. Nat Methods 17, 913–916 (2020). https://doi.org/10.1038/s41592-020-0882-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-020-0882-0
This article is cited by
-
Label-free Brillouin endo-microscopy for the quantitative 3D imaging of sub-micrometre biology
Communications Biology (2024)
-
Brillouin microscopy
Nature Reviews Methods Primers (2024)
-
Corneal biomechanics and diagnostics: a review
International Ophthalmology (2024)
-
High-resolution line-scan Brillouin microscopy for live imaging of mechanical properties during embryo development
Nature Methods (2023)
-
Pulsed stimulated Brillouin microscopy enables high-sensitivity mechanical imaging of live and fragile biological specimens
Nature Methods (2023)