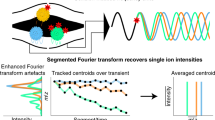
Abstract
We demonstrate single-particle charge detection mass spectrometry on an Orbitrap for the analysis of megadalton biomolecular assemblies. We establish that the signal amplitudes of individual ions scale linearly with their charge, which can be used to resolve mixed ion populations, determine charge states and thus also determine the masses of individual ions. This enables the ultrasensitive analysis of heterogeneous protein assemblies including immunoglobulin oligomers, ribosomes, proteinaceous nanocontainers and genome-packed adeno-associated viruses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
For each of the figures and supplementary figures, the underlying centroided and filtered data are available in Supplementary Data. On request, the raw data are available from the corresponding author.
Code availability
A python class and an exemplary script for single-particle data processing are available as Supplementary Software.
References
Leney, A. C. & Heck, A. J. R. Native mass spectrometry: what is in the name? J. Am. Soc. Mass Spectrom. 28, 5–13 (2017).
Mehmood, S., Allison, T. M. & Robinson, C. V. Mass spectrometry of protein complexes: from origins to applications. Annu. Rev. Phys. Chem. 66, 453–474 (2015).
Snijder, J. & Heck, A. J. R. Analytical approaches for size and mass analysis of large protein assemblies. Annu. Rev. Anal. Chem. 7, 43–64 (2014).
Mann, M., Meng, C. K. & Fenn, J. B. Interpreting mass spectra of multiply charged ions. Anal. Chem. 61, 1702–1708 (1989).
Chang, H.-C. Ultrahigh-mass mass spectrometry of single biomolecules and bioparticles. Annu. Rev. Anal. Chem. 2, 169–185 (2010).
Keifer, D. Z. & Jarrold, M. F. Single-molecule mass spectrometry. Mass Spectrom. Rev. 36, 715–733 (2017).
Hanay, M. S. et al. Single-protein nanomechanical mass spectrometry in real time. Nat. Nanotechnol. 7, 602–608 (2012).
Sage, E. et al. Neutral particle mass spectrometry with nanomechanical systems. Nat. Commun. 6, e6482 (2015).
Elliott, A. G. et al. Simultaneous measurements of mass and collisional cross-section of single ions with charge detection mass spectrometry. Anal. Chem. 89, 7701–7708 (2017).
Doussineau, T. et al. Mass determination of entire amyloid fibrils by using mass spectrometry. Angew. Chem. - Int. Ed. 55, 2340–2344 (2016).
Pierson, E. E., Keifer, D. Z., Asokan, A. & Jarrold, M. F. Resolving adeno-associated viral particle diversity with charge detection mass spectrometry. Anal. Chem. 88, 6718–6725 (2016).
Dominguez-Medina, S. et al. Neutral mass spectrometry of virus capsids above 100 megadaltons with nanomechanical resonators. Science 362, 918–922 (2018).
Rose, R. J., Damoc, E., Denisov, E., Makarov, A. & Heck, A. J. R. High-sensitivity Orbitrap mass analysis of intact macromolecular assemblies. Nat. Methods 9, 1084–1086 (2012).
Makarov, A. & Denisov, E. Dynamics of ions of intact proteins in the Orbitrap mass analyzer. J. Am. Soc. Mass Spectrom. 20, 1486–1495 (2009).
Kafader, J. O. et al. Measurement of individual ions sharply increases the resolution of Orbitrap mass spectra of proteins. Anal. Chem. 91, 2776–2783 (2019).
Bruce, J. E. et al. Trapping, detection, and mass measurement of individual ions in a Fourier transform ion cyclotron resonance mass spectrometer. J. Am. Chem. Soc. 116, 7839–7847 (1994).
Sage, E. et al. Single-particle mass spectrometry with arrays of frequency-addressed nanomechanical resonators. Nat. Commun. 9, 3283 (2018).
Pacholarz, K. J. & Barran, P. E. Use of a charge reducing agent to enable intact mass analysis of cysteine-linked antibody-drug-conjugates by native mass spectrometry. EuPA Open Proteom. 11, 23–27 (2016).
Hiramoto, E. et al. The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci. Adv. 4, eaau1199 (2018).
Snijder, J. et al. Defining the stoichiometry and cargo load of viral and bacterial nanoparticles by orbitrap mass spectrometry. J. Am. Chem. Soc. 136, 7295–7299 (2014).
van de Waterbeemd, M. et al. High-fidelity mass analysis unveils heterogeneity in intact ribosomal particles. Nat. Methods 14, 283–286 (2017).
Fort, K. L. et al. Expanding the structural analysis capabilities on an Orbitrap-based mass spectrometer for large macromolecular complexes. Analyst 143, 100–105 (2017).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012).
Veesler, D. et al. Architecture of a dsDNA viral capsid in complex with its maturation protease. Structure 22, 230–237 (2014).
Sigmund, F. et al. Bacterial encapsulins as orthogonal compartments for mammalian cell engineering. Nat. Commun. 9, e1990 (2018).
Acknowledgements
We greatly appreciate the several collaborators who provided samples to us that we used in the work presented here. We acknowledge the D. Hilvert group at the ETH Zurich for providing the AaLS-neg samples, the J. Schuurman group at Genmab for providing the mutant IgG samples, the S. Rooijakkers group at the Medical Microbiology Department, University Medical Center, Utrecht, in particular P. Aerts and C. Gosselaar-de Haas, for providing the IgM samples, and the A. Routh group at the University of Texas Medical Branch, in particular E. Jaworski, for providing the FHV samples. This work was supported by the Netherlands Organization for Scientific Research through the Spinoza Award (no. SPI.2017.028) to A.J.R.H. J.S. was further supported by the NWO Gravitation project Institute for Chemical Immunology (no. 024.002.009). Additional support came through the European Union Horizon 2020 INFRAIA project Epic-XS (project no. 823839).
Author information
Authors and Affiliations
Contributions
T.P.W., J.S. and A.J.R.H. conceived the project, designed the experiments and wrote the paper. A.B. and M.A.-M. prepared the AAV samples. A.A.M. advised on operation of data acquisition system and mechanisms of ion–molecule interactions in the Orbitrap analyzer for high mass ions. T.P.W. performed all experiments and processed the data. J.S. and A.J.R.H. supervised the project. T.P.W., J.S. and A.J.R.H. analyzed the results. All authors discussed the results and edited the paper.
Corresponding author
Ethics declarations
Competing interests
A.A.M. is an employee of Thermo Fisher Scientific, the company that commercializes Orbitrap-based mass analyzers.
Additional information
Peer review information Allison Doerr was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Pressure dependent ion dephasing for the AaLS-neg nanocontainers (Mw ~ 3MDa, z ~ 145).
2D histograms for AaLS-neg ions acquired at different pressure settings before filtering. Nitrogen was used as collision gas and the readout of the ultra-high vacuum cold cathode gauge is shown, together with the percentage of ions that remain stable over the full transient, above each histogram. These stable ions are located between the dotted lines, indicating the expected intensity for ions surviving the whole set transient time of 1,024 ms. The trailing of the ion intensities towards lower values is primarily caused by peak splitting, presumably resulting from dephasing and/or mass loss of particles during the transient. Both effects are expected to originate from collisions with background gas and/or metastable decay during detection and increase at elevated pressure.
Supplementary Figure 2 Single particle resolution.
Extracted mass resolution for single particle events (n=124) extracted from the data shown in Fig. 1 of the main text. Peak broadening causes some centroids to appear at slightly lower values than the theoretical resolution. Peak broadening can be caused by solvent loss during acquisition or if the ion does not survive over the set transient time. The theoretical resolution was obtained by fitting the dependency: Resolution ~ m/z-1/2. The resulting resolution at 400 m/z is in close agreement with the reported values from the manufacturer for a 1,024 ms transient (213,235 vs. 200,000 at 400 m/z).
Supplementary Figure 3 General procedure for establishing Intensity vs. Charge regression model shown for AaLS-neg, (z=149).
A Single particle centroids, preprocessed and filtered as described in Supplementary Fig. 8, were binned in m/z and smoothed. The resulting mass spectrum was used for conventional charge state assignment. B Centroids were filtered for charge state 149 by only allowing centroids within 80 Th of the top peak position to pass the filtering. C Filtered centroids were subjected to a kernel density estimation and the most abundant intensity as well as the FWHM of the distribution were extracted. D To obtain an equal weighted fit over all tested charges 200 random samples were drawn for each charge within two times the FWHM of the most abundant intensity. Assuming a normal distribution this contains more than 98 % of the centroids (FWHM = 2.35σ). E & F The same procedure was repeated for the other examined proteins and charges and used for establishing the linear regression model.
Supplementary Figure 4 Resolving mixed ion populations with overlapping m/z based on single ion intensities.
A Individual scans of single particles of AaLS-neg (left), 50S E. coli ribosomal particles (middle) (3.05 and 1.39 MDa, respectively) and a mixture of both these particles (right). Ion intensities display the expected intensity of ≈1.8*103 for AaLS-neg and ≈0.8*103 for the 50S particles. B Corresponding binned 2D histogram of larger datasets of single particles. Single ion signals can be easily assigned to AaLS-neg or 50S based on intensity, even within the same scan.
Supplementary Figure 5 Resolving IgG oligomers based on single ion intensities.
A Conventional native MS spectrum of IgG1-RGY. B Detailed view of the spectrum in A, showing the exact overlap between two charge states from the trimer (42+) and tetramer (56+) distributions at 10,649 m/z. C Single particle CD-MS spectrum of IgG1-RGY. D. Detailed view of the same region as in B, showing complete resolution of the coinciding trimer and tetramer charge states in the intensity dimension.
Supplementary Figure 6 Dynamic range in single particle experiments.
A 2D histogram of IgG1-RGY as shown in Supplemental Fig. 5C. To estimate the dynamic range within one experiment we compared the ion counts of the highest and lowest detectable charge states, for the highest and lowest abundant IgG oligomer, respectively. Panels B and C show an expansion of the respective region indicated with dotted lines in A. The selected charge states are shown in the 1D histogram above the expansion for B and C. Comparing the ion counts for charge state +47 of the trimeric (1536) and +49 of the tetrameric (11) IgG oligomer, yields a dynamic range of ~150, whereby the 11 ions for the charge state +49 only account for around 0.05 % of the total number of ions displayed in A.
Supplementary Figure 7 m/z position based mass prediction.
A Mass spectrum of AAV8 recorded on an QToF mass spectrometer modified for high mass measurements. Peak positions for empty and genome loaded AAV8 are indicated with empty and solid blue circles (24,012 m/z and 29,459 m/z, respectively). B Empirical dependency between m/z position and mass for 76 globular proteins, measured and reported previously, fitted with an exponential function a,b. The resulting formula Mass[kDa] = 1.63*10-6 * m/z2.14 was used to calculate the mass for the empty and genome loaded AAV8 particle (3,764 kDa and 5,828 kDa respectively). The calculated mass for the empty AAV8 is in close agreement with the expected mass but the mass of the genome loaded particle deviates by ~1 MDa. a: Veesler, D. et al. Architecture of a dsDNA viral capsid in complex with its maturation protease. Structure 22, 230–237 (2014). b: Sigmund, F. et al. Bacterial encapsulins as orthogonal compartments for mammalian cell engineering. Nat. Commun. 9, (2018).
Supplementary Figure 8 General workflow for single particle pre-processing.
A Data was acquired on a QE-UHMR. For the data shown in this work, scans were collected for 10 to 60 minutes. After acquisition, .RAW files were centroided and converted into the mzXML format. B Centroids were subjected to filtering. We rejected all centroids which were below a certain intensity value as well as centroids which have an adjacent peak within a certain m/z threshold above the noise limit (See red asterisk in rejected traces). C&D Effect of filtering on AaLS-neg dataset. C 2D histogram of single particle centroids before filtering. Ion intensities trail toward lower values, which is mainly attributed to the presence of split peaks (see Supplementary Fig. 1). D Dataset after noise filtering and removal of adjacent peaks. Filtered single particle centroids are more confined in the intensity domain and don’t show the trailing towards lower intensities anymore.
Supplementary Figure 9 Linear regression models for Intensity vs. Charge correlation.
A Linear regression models (n=15,600) for the data shown in the main text. All data was recorded within three days. Data was fitted using a general linear model (red line) as well as one forced through the origin (black dashed line). Both regression models gave fits of similar quality (∆r2=2*10-6). The intersection at zero of the general regression model translates to less than 0.28 charges, hence we used for this work the one forced through the origin. B Technical replicate of the Intensity vs. Charge correlation (n=12,400) shown in the main text and A. In contrast to A, data was acquired over a period of three months and at mixed resolution settings (512 ms and 1,024 ms). The maximum deviation between all shown regression models doesn’t exceed 1.6 charges over the displayed range (1 to 250 charges).
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Note and Table 1.
Supplementary Software
Python class and exemplary script for single-particle data processing.
Supplementary Data
Source data for the figures and supplementary figures. The corresponding folder for each figure or supplementary figure contains the displayed single-particle centroids and native MS spectra, as well as other displayed data.
Rights and permissions
About this article
Cite this article
Wörner, T.P., Snijder, J., Bennett, A. et al. Resolving heterogeneous macromolecular assemblies by Orbitrap-based single-particle charge detection mass spectrometry. Nat Methods 17, 395–398 (2020). https://doi.org/10.1038/s41592-020-0770-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-020-0770-7
This article is cited by
-
Ultralong transients enhance sensitivity and resolution in Orbitrap-based single-ion mass spectrometry
Nature Methods (2024)
-
Artificial surface labelling of Escherichia coli with StrepTagII antigen to study how monoclonal antibodies drive complement-mediated killing
Scientific Reports (2023)
-
Mass spectrometry using electrospray ionization
Nature Reviews Methods Primers (2023)
-
Sampling the proteome by emerging single-molecule and mass spectrometry methods
Nature Methods (2023)
-
High-throughput determination of dry mass of single bacterial cells by ultrathin membrane resonators
Communications Biology (2022)