Abstract
We report a molecular assay, Methyl-HiC, that can simultaneously capture the chromosome conformation and DNA methylome in a cell. Methyl-HiC reveals coordinated DNA methylation status between distal genomic segments that are in spatial proximity in the nucleus, and delineates heterogeneity of both the chromatin architecture and DNA methylome in a mixed population. It enables simultaneous characterization of cell-type-specific chromatin organization and epigenome in complex tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Figures show merged data from all replicates. The bulk Methyl-HiC, single-cell Methyl-HiC and WGBS datasets generated in this study have been deposited in the Gene Expression Omnibus (GEO) under the accession number GSE119171. Previously published data used for this study are listed in the Supplementary Notes.
Code availability
All of the source code for Bhmem software is publicly available at Bitbucket via https://bitbucket.org/dnaase/bisulfitehic/src/master/
References
Iurlaro, M., von Meyenn, F. & Reik, W. Curr. Opin. Genet. Dev. 43, 101–109 (2017).
Lyko, F. Nat. Rev. Genet. 19, 81–92 (2018).
Guo, J. U., Su, Y., Zhong, C., Ming, G.-l & Song, H. Cell 145, 423–434 (2011).
Luo, C. et al. Science 357, 600 (2017).
Lister, R. et al. Nature 462, 315 (2009).
Guo, S. et al. Nat. Genet. 49, 635 (2017).
Shoemaker, R., Deng, J., Wang, W. & Zhang, K. Genome Res. 20, 883–889 (2010).
Dixon, JesseR., Gorkin, DavidU. & Ren, B. Mol. Cell 62, 668–680 (2016).
Lieberman-Aiden, E. et al. Science 326, 289 (2009).
Dixon, J. R. et al. Nature 485, 376 (2012).
Rao, SuhasS. P. et al. Cell 159, 1665–1680 (2014).
Schmitt, A. D., Hu, M. & Ren, B. Nat. Rev. Mol. Cell Biol. 17, 743 (2016).
Kubo, N. et al. Preprint at bioRxiv https://doi.org/10.1101/118737 (2017).
Schwartzman, O. & Tanay, A. Nat. Rev. Genet. 16, 716 (2015).
Nagano, T. et al. Nature 547, 61–67 (2017).
Flyamer, I. M. et al. Nature 544, 110 (2017).
Ramani, V. et al. Nat. Methods 14, 263–266 (2017).
Smallwood, S. A. et al. Nat. Methods 11, 817 (2014).
Rodríguez-Carballo, E. et al. Genes Dev. 31, 2264–2281 (2017).
Liu, Y. et al. Nat. Biotechnol. 37, 424–429 (2019).
Diedenhofen, B. & Musch, J. PLoS ONE 10, e0121945 (2015).
Yang, T. et al. Genome Res. 27, 1939–1949 (2017).
Gribnau, J., Hochedlinger, K., Hata, K., Li, E. & Jaenisch, R. Genes Dev. 17, 759–773 (2003).
Durand, N. C. et al. Cell Syst. 3, 99–101 (2016).
Liu, Y., Siegmund, K. D., Laird, P. W. & Berman, B. P. Genome Biol. 13, R61 (2012).
Acknowledgements
We thank S. Kuan and B. Li for processing of sequencing. The study was supported in part by the NIH 4D Nucleome program (grant no. U54DK107977) (to B.R.), grant no. 1U01HG007610-01 (to M.K.) and a start-up grant from CCHMC (to Y.L.).
Author information
Authors and Affiliations
Contributions
B.R., G.L. and Y.L. conceived the study and prepared the manuscript. G.L. performed the Methyl-HiC experiments. Y.L. wrote the bioinformatics pipeline and performed the analysis with the guidance of M.K. Y.L., G.L. and Y.Z. performed the in situ Hi-C and WGBS analyses. M.Y. and R.F. helped on single-cell Hi-C data analysis. N.K. provided the list of loops with CTCF. G.L., Y.L. and B.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.R. is a cofounder of Arima Genomics, Inc.
Additional information
Peer review information: Nicole Rusk was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
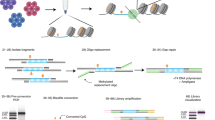
Supplementary Figure 1 Methyl-HiC simultaneously profiles chromatin architecture and DNA methylome in the mouse embryonic stem cells.
a. The workflow of Methyl-HiC. Biotin-enriched DNA fragments from in situ Hi-C are bisulfite converted followed by paired-end sequencing. The short sequencing reads are mapped to the genome and processed by in-house computational pipeline Bhmem. b. A flowchart of the Bhmem pipeline for mapping and analysis of the Methyl-HiC data. c. Bhmem maps the sequence reads to the reference mouse genome as accurately as Bismark. Sherman simulated bisulfite converted reads were used in the comparison. d. Methylation levels of simulated Hi-C and bisulfite converted reads by different pipelines. Reads were simulated by sim3C1 and in silica bisulfite conversion. e. Bar chart shows the percentages of sequence reads in the Methyl-HiC dataset mapped to the reference genome by Bismark and Bhmem. As a reference, the percentage of sequence reads in a regular in situ Hi-C dataset is provided. f. The complexity of DNA libraries obtained from Methyl-HiC and in situ Hi-C is similar. The library complexity was evaluated by preseq software that predicts the distinct reads from initial sequencing libraries.
Supplementary Figure 2 Methyl-HiC performance compare to in situ Hi-C and WGBS.
a. The overlapping of HiCCUPS loop from in situ Hi-C and Methyl-HiC. b. Enrichment of enhancers and promoters with active histone markers and CTCF binding on HiCCUPS loop anchors at 25kb resolution (n=3110) called from Methyl-HiC dataset. Significance was assessed using Fisher’s exact test. c. The overlapping of TADs from in situ Hi-C and Methyl-HiC. d. Distribution of TADs size in in situ Hi-C (n=2165) and Methyl-HiC (n=1561), with same median size of 1,150,000 bp. Boxes are median with interquartile. e. Overlapping of CpG sites covered in WGBS and Methyl-HiC. f. The distributions of CpGs from WGBS and Methyl-HiC datasets in various chromatin states.
Supplementary Figure 3 DNA methylation concordance between spatially proximal regions.
a. Genome-wide DNA methylation correlation calculated in loop anchors by overlapping loops (n=3000) from in situ Hi-C and WGBS datasets from the same cell line (Hi-C+WGBS) or by overlapping loops (n=3110) and DNA methylation tracks from the same experiment (Methyl-HiC). Significance were assessed by Pearson Correlation Coefficient. b. Illustration of the calculation for methylation concordance on DNA loop anchors. Only reads have CpGs on both ends are considered. Read ends locate in anchor regions but are not linked are used as controls. c. An illustration shows the methylation status on read pairs supporting a chromatin loop. Methylation status of each end is determined by the average methylation level of CpG sites on the reads. d. Pearson correlation coefficient distribution of individual loops (n=3110). Boxes are median with quartiles and whiskers extend to 5th and 95th percentile. e. DNA methylation concordance comparison between loops with both anchors overlapping with CTCF peaks (n=2,837) and non-CTCF loops (n=3,145). Significance was assessed using Fisher’s (1925) z in cocor package. f. DNA methylation concordance comparison between reads (n=167,464) with both ends originated from the same TADs and reads (n=52,675) from two different TADs. Only reads containing 2 or more CpGs on each end were included. Significance was assessed using Fisher’s (1925) z in cocor package. g. DNA methylation concordance comparison between reads with both ends originated from compartment A (n=115,286) and B (n=218,488). Only reads containing 2 or more CpGs on each end were included. Significance was assessed using Fisher’s (1925) z in cocor package. h. Pearson correlation coefficient of DNA methylation concordance between 1Mb bins from different chromosomes. Inter-chromosomal reads connecting every 1Mb bins in two chromosomes (n=773,026) were extracted and methylation concordance was calculated. Paired reads located within the same bins were shuffled and used to calculate the expected values. Only reads containing 2 or more CpGs on each end were included. Significance was assessed using Fisher’s (1925) z in cocor package.
Supplementary Figure 4 DNA methylation concordance in enhancers and promoters.
a. Emission parameters of ChromHMM states related to enhancers and promoters. b. Pearson correlation coefficient of DNA methylation concordance between anchors of chromatin loops within various chromatin states related to enhancers and promoters. Read ends contain at least 2 CpGs were used to calculate methylation concordance. NS means not significant (p-value > 0.05). c. Pearson Correlation Coefficient of DNA methylation concordance between various chromatin states related to promoter and enhancer. Read ends contain at least 2 CpGs were used to calculate methylation concordance. The numbers of useful reads are marked in the bar.
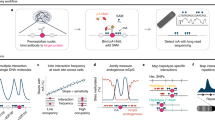
Supplementary Figure 5 Simultaneous analysis of DNA methylome and chromatin architecture in individual cells by Single Cell Methyl-HiC.
a. Contact probability of merged single cell Methyl-HiC and bulk Methyl-HiC data. b. Genome wide contact matrix comparison between resembled single cell Methyl-HiC data and bulk Methyl-HiC data. c. Pearson’s correlation matrix comparison between ensemble single cell Methyl-HiC data (n=103) and bulk Methyl-HiC data. PC1 was plotted on top. d. Contact matrixes comparison between randomly selected replications (n=50) from ensemble single cell Hi-C3 and single cell Methyl-HiC for mouse embryonic stem cells in serum condition. 50 cells are randomly selected for each replication to plot the matrixes at 1Mb resolution. Matrix similarity is measured by HiCrep. SCC means stratum-adjusted correlation coefficient.
Supplementary Figure 6 Single cell Methyl-HiC reveals heterogeneity of cultured mouse embryonic stem cells.
a. Overlapping of compartments between 3 clusters. b. The distribution of compartments (column 1 and 2), average DNA methylation level in each compartment (column 3 and 4), and number of DMRs in each compartment between Cluster 2 and Cluster 3 (column 5). c. Distribution of DMRs between compartments in Cluster 2 and Cluster 3. d. Distribution of DMRs between clusters in different chromosome states. e. The Pearson’s correlation matrixes around HoxD gene clusters and EphA4 gene in Cluster 2 (n=26) and Cluster 3 (n=24). The chromosome-wide matrixes are in 1Mb resolution. Dotted squares are magnified to show the nearby regions. Squares in the magnified fields show the bins where the HoxD gene clusters and Epha4 locate. f. The snapshot for Epha4 gene in different compartments between Cluster 2 and Cluster 3. Hi-C matrix are from bulk serum mESCs to show the overall chromosome organization around Epha4 genes. The two tracks below Hi-C matrix show the eigenvector decomposition, in which the positive/negative values correspond to compartment A/B, respectively.
Supplementary information
Supplementary Information
Supplementary Figures 1–6, Supplementary Note and Supplementary Protocol.
Rights and permissions
About this article
Cite this article
Li, G., Liu, Y., Zhang, Y. et al. Joint profiling of DNA methylation and chromatin architecture in single cells. Nat Methods 16, 991–993 (2019). https://doi.org/10.1038/s41592-019-0502-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-019-0502-z
This article is cited by
-
Simultaneous single-cell analysis of 5mC and 5hmC with SIMPLE-seq
Nature Biotechnology (2024)
-
Advances in single-cell omics and multiomics for high-resolution molecular profiling
Experimental & Molecular Medicine (2024)
-
Mosaic integration and knowledge transfer of single-cell multimodal data with MIDAS
Nature Biotechnology (2024)
-
Single-cell sequencing technology applied to epigenetics for the study of tumor heterogeneity
Clinical Epigenetics (2023)
-
A novel approach to risk exposure and epigenetics—the use of multidimensional context to gain insights into the early origins of cardiometabolic and neurocognitive health
BMC Medicine (2023)