Abstract
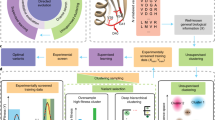
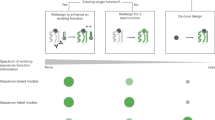
Protein engineering through machine-learning-guided directed evolution enables the optimization of protein functions. Machine-learning approaches predict how sequence maps to function in a data-driven manner without requiring a detailed model of the underlying physics or biological pathways. Such methods accelerate directed evolution by learning from the properties of characterized variants and using that information to select sequences that are likely to exhibit improved properties. Here we introduce the steps required to build machine-learning sequence–function models and to use those models to guide engineering, making recommendations at each stage. This review covers basic concepts relevant to the use of machine learning for protein engineering, as well as the current literature and applications of this engineering paradigm. We illustrate the process with two case studies. Finally, we look to future opportunities for machine learning to enable the discovery of unknown protein functions and uncover the relationship between protein sequence and function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dou, J. et al. Sampling and energy evaluation challenges in ligand binding protein design. Protein Sci. 26, 2426–2437 (2017).
Garcia-Borras, M., Houk, K. N. & Jiménez-Osés, G. Computational design of protein function. In Computational Tools for Chemical Biology (ed. Martín-Santamaría, S.) 87–107 (Royal Society of Chemistry, 2017).
Mandecki, W. The game of chess and searches in protein sequence space. Trends Biotechnol. 16, 200–202 (1998).
Pierce, N. A. & Winfree, E. Protein design is NP-hard. Protein Eng. 15, 779–782 (2002).
Smith, J. M. Natural selection and the concept of a protein space. Nature 225, 563–564 (1970).
Orr, H. A. The distribution of fitness effects among beneficial mutations in Fisher’s geometric model of adaptation. J. Theor. Biol. 238, 279–285 (2006).
Khersonsky, O. & Tawfik, D. S. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu. Rev. Biochem. 79, 471–505 (2010).
Romero, P. A. & Arnold, F. H. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 10, 866–876 (2009).
Drummond, D. A., Silberg, J. J., Meyer, M. M., Wilke, C. O. & Arnold, F. H. On the conservative nature of intragenic recombination. Proc. Natl Acad. Sci. USA 102, 5380–5385 (2005).
Fox, R. J. et al. Improving catalytic function by ProSAR-driven enzyme evolution. Nat. Biotechnol. 25, 338–344 (2007).
Romero, P. A., Krause, A. & Arnold, F. H. Navigating the protein fitness landscape with Gaussian processes. Proc. Natl Acad. Sci. USA 110, E193–E201 (2013). This is the first study to combine SCHEMA recombination with the GP-UCB algorithm to optimize a protein property.
Bedbrook, C. N., Yang, K. K., Rice, A. J., Gradinaru, V. & Arnold, F. H. Machine learning to design integral membrane channelrhodopsins for efficient eukaryotic expression and plasma membrane localization. PLoS Comput. Biol. 13, e1005786 (2017).
Bedbrook, C. N., Yang, K. K., Robinson, J. E., Gradinaru, V. & Arnold, F. H. Machine learning-guided channelrhodopsin engineering enables minimally-invasive optogenetics. Preprint at https://www.biorxiv.org/content/10.1101/565606v1 (2019). This paper demonstrates the utility of machine learning for optimizing a property that would not be possible to engineer with directed evolution alone.
Wu, Z., Kan, S. B. J., Lewis, R. D., Wittmann, B. J. & Arnold, F. H. Machine learning-assisted directed protein evolution with combinatorial libraries. Proc. Natl Acad. Sci. USA 116, 8852–8858 (2019).
Hastie, T. & Tibshirani, R. The Elements of Statistical Learning: Data Mining, Inference and Prediction (Springer, 2008).
Murphy, K. Machine Learning, a Probabilistic Perspective (MIT Press, 2012). Murphy’s textbook provides a thorough introduction to modern machine learning.
Liao, J. et al. Engineering proteinase K using machine learning and synthetic genes. BMC Biotechnol. 7, 16 (2007).
Govindarajan, S. et al. Mapping of amino acid substitutions conferring herbicide resistance in wheat glutathione transferase. ACS Synth. Biol. 4, 221–227 (2015).
Musdal, Y., Govindarajan, S. & Mannervik, B. Exploring sequence-function space of a poplar glutathione transferase using designed information-rich gene variants. Protein Eng. Des. Sel. 30, 543–549 (2017).
Wolpert, D. H. The lack of a priori distinctions between learning algorithms. Neural Comput. 8, 1341–1390 (1996).
Li, Y. et al. A diverse family of thermostable cytochrome P450s created by recombination of stabilizing fragments. Nat. Biotechnol. 25, 1051–1056 (2007).
Breiman, L. Classification and Regression Trees (Routledge, 2017).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Friedman, J. H. Stochastic gradient boosting. Comput. Stat. Data Anal. 38, 367–378 (2002).
Tian, J., Wu, N., Chu, X. & Fan, Y. Predicting changes in protein thermostability brought about by single- or multi-site mutations. BMC Bioinforma. 11, 370 (2010).
Li, Y. & Fang, J. PROTS-RF: a robust model for predicting mutation-induced protein stability changes. PLoS One 7, e47247 (2012).
Jia, L., Yarlagadda, R. & Reed, C. C. Structure based thermostability prediction models for protein single point mutations with machine learning tools. PLoS One 10, e0138022 (2015).
Cortes, C. & Vapnik, V. Support-vector networks. Mach. Learn. 20, 273–297 (1995).
Nadaraya, E. On estimating regression. Theory Probab. Its Appl. 9, 141–142 (1964).
Leslie, C., Eskin, E. & Noble, W. S. The spectrum kernel: a string kernel for SVM protein classification. Pac. Symp. Biocomput. 2002, 564–575 (2002).
Leslie, C. S., Eskin, E., Cohen, A., Weston, J. & Noble, W. S. Mismatch string kernels for discriminative protein classification. Bioinformatics 20, 467–476 (2004).
Jokinen, E., Heinonen, M. & Lähdesmäki, H. mGPfusion: predicting protein stability changes with Gaussian process kernel learning and data fusion. Bioinformatics 34, i274–i283 (2018).
Capriotti, E., Fariselli, P. & Casadio, R. I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 33, W306–W310 (2005).
Capriotti, E., Fariselli, P., Calabrese, R. & Casadio, R. Predicting protein stability changes from sequences using support vector machines. Bioinformatics 21, ii54–ii58 (2005).
Cheng, J., Randall, A. & Baldi, P. Prediction of protein stability changes for single-site mutations using support vector machines. Proteins 62, 1125–1132 (2006).
Buske, F. A., Their, R., Gillam, E. M. & Bodén, M. In silico characterization of protein chimeras: relating sequence and function within the same fold. Proteins 77, 111–120 (2009).
Liu, J. & Kang, X. Grading amino acid properties increased accuracies of single point mutation on protein stability prediction. BMC Bioinforma. 13, 44 (2012).
Zaugg, J., Gumulya, Y., Malde, A. K. & Bodén, M. Learning epistatic interactions from sequence-activity data to predict enantioselectivity. J. Comput. Aided Mol. Des. 31, 1085–1096 (2017).
Saladi, S. M., Javed, N., Müller, A. & Clemons, W. M. Jr. A statistical model for improved membrane protein expression using sequence-derived features. J. Biol. Chem. 293, 4913–4927 (2018).
Rasmussen, C. E. & Williams, C. K. I. Gaussian Processes for Machine Learning (MIT Press, 2006).
Wilson, A. G. & Nickisch, H. Kernel interpolation for scalable structured Gaussian processes (KISS-GP). In Proc. 32nd International Conference on Machine Learning (eds. Bach, F. & Blei, D.) 1775–1784 (JMLR, 2015).
Wang, K. A. et al. Exact Gaussian processes on a million data points. Preprint at https://arxiv.org/abs/1903.08114 (2019).
Pires, D. E., Ascher, D. B. & Blundell, T. L. mCSM: predicting the effects of mutations in proteins using graph-based signatures. Bioinformatics 30, 335–342 (2014).
Mellor, J., Grigoras, I., Carbonell, P. & Faulon, J.-L. Semisupervised Gaussian process for automated enzyme search. ACS Synth. Biol. 5, 518–528 (2016).
Saito, Y. et al. Machine-learning-guided mutagenesis for directed evolution of fluorescent proteins. ACS Synth. Biol. 7, 2014–2022 (2018).
Zhang, S. et al. A deep learning framework for modeling structural features of RNA-binding protein targets. Nucleic Acids Res. 44, e32 (2016).
Alipanahi, B., Delong, A., Weirauch, M. T. & Frey, B. J. Predicting the sequence specificities of DNA- and RNA-binding proteins by deep learning. Nat. Biotechnol. 33, 831–838 (2015).
Zeng, H., Edwards, M. D., Liu, G. & Gifford, D. K. Convolutional neural network architectures for predicting DNA-protein binding. Bioinformatics 32, i121–i127 (2016).
Hu, J. & Liu, Z. DeepMHC: deep convolutional neural networks for high-performance peptide-MHC binding affinity prediction. Preprint at https://www.biorxiv.org/content/early/2017/12/24/239236 (2017).
Jiménez, J., Doerr, S., Martínez-Rosell, G., Rose, A. S. & De Fabritiis, G. DeepSite: protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 33, 3036–3042 (2017).
Gomes, J., Ramsundar, B., Feinberg, E. N. & Pande, V. S. Atomic convolutional networks for predicting protein-ligand binding affinity. Preprint at https://arxiv.org/abs/1703.10603 (2017).
Mazzaferro, C. Predicting protein binding affinity with word embeddings and recurrent neural networks. Preprint at https://www.biorxiv.org/content/early/2017/04/18/128223 (2017).
Khurana, S. et al. DeepSol: a deep learning framework for sequence-based protein solubility prediction. Bioinformatics 34, 2605–2613 (2018).
Dehouck, Y. et al. Fast and accurate predictions of protein stability changes upon mutations using statistical potentials and neural networks: PoPMuSiC-2.0. Bioinformatics 25, 2537–2543 (2009).
Giollo, M., Martin, A. J., Walsh, I., Ferrari, C. & Tosatto, S. C. NeEMO: a method using residue interaction networks to improve prediction of protein stability upon mutation. BMC Genom. 15, S7 (2014).
Almagro Armenteros, J. J., Sønderby, C. K., Sønderby, S. K., Nielsen, H. & Winther, O. DeepLoc: prediction of protein subcellular localization using deep learning. Bioinformatics 33, 3387–3395 (2017).
Sønderby, S. K. & Winther, O. Protein secondary structure prediction with long short term memory networks. Preprint at https://arxiv.org/abs/1412.7828 (2014).
Szalkai, B. & Grolmusz, V. Near perfect protein multi-label classification with deep neural networks. Methods 132, 50–56 (2018).
Cao, R. et al. ProLanGO: protein function prediction using neural machine translation based on a recurrent neural network. Molecules 22, 1732 (2017).
Bileschi, M. L. et al. Using deep learning to annotate the protein universe. Preprint at https://www.biorxiv.org/content/10.1101/626507v3 (2019).
Hopf, T. A. et al. Three-dimensional structures of membrane proteins from genomic sequencing. Cell 149, 1607–1621 (2012).
Snoek, J., Larochelle, H. & Adams, R. P. Practical Bayesian optimization of machine learning algorithms. In NIPS ’12: Proceedings of the 25th International Conference on Neural Information Processing Systems (eds. Pereira, F. et al.) 2951–2959 (Curran Associates, 2012).
Domingos, P. A few useful things to know about machine learning. Commun. ACM 55, 78–87 (2012).
Bengio, Y., Courville, A. & Vincent, P. Representation learning: a review and new perspectives. IEEE Trans. Pattern Anal. Mach. Intell. 35, 1798–1828 (2013).
Kawashima, S. et al. AAindex: amino acid index database, progress report 2008. Nucleic Acids Res. 36, D202–D205 (2008).
Ofer, D. & Linial, M. ProFET: feature engineering captures high-level protein functions. Bioinformatics 31, 3429–3436 (2015).
Barley, M. H., Turner, N. J. & Goodacre, R. Improved descriptors for the quantitative structure–activity relationship modeling of peptides and proteins. J. Chem. Inf. Model. 58, 234–243 (2018).
Qiu, J., Hue, M., Ben-Hur, A., Vert, J.-P. & Noble, W. S. A structural alignment kernel for protein structures. Bioinformatics 23, 1090–1098 (2007).
Henikoff, S. & Henikoff, J. G. Amino acid substitution matrices from protein blocks. Proc. Natl Acad. Sci. USA 89, 10915–10919 (1992).
Asgari, E. & Mofrad, M. R. Continuous distributed representation of biological sequences for deep proteomics and genomics. PLoS One 10, e0141287 (2015).
Ng, P. dna2vec: consistent vector representations of variable-length k-mers. Preprint at https://arxiv.org/abs/1701.06279 (2017).
Kimothi, D., Soni, A., Biyani, P. & Hogan, J. M. Distributed representations for biological sequence analysis. Preprint at https://arxiv.org/abs/1608.05949 (2016).
Yang, K. K., Wu, Z., Bedbrook, C. N. & Arnold, F. H. Learned protein embeddings for machine learning. Bioinformatics 34, 2642–2648 (2018).
Schwartz, A. S. et al. Deep semantic protein representation for annotation, discovery, and engineering. Preprint at https://www.biorxiv.org/content/early/2018/07/10/365965 (2018).
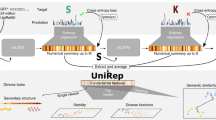
Alley, E. C., Khimulya, G., Biswas, S., AlQuraishi, M. & Church, G. M. Unified rational protein engineering with sequence-only deep representation learning. Preprint at https://www.biorxiv.org/content/10.1101/589333v1 (2019).
Rives, A. et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Preprint at https://www.biorxiv.org/content/10.1101/622803v2 (2019).
Bepler, T. & Berger, B. Learning protein sequence embeddings using information from structure. Seventh International Conference on Learning Representations https://openreview.net/forum?id=SygLehCqtm (2019).
Yang, K. K., Chen, Y., Lee, A. & Yue, Y. Batched stochastic Bayesian optimization via combinatorial constraints design. Proc. Mach. Learn. Res. 89, 3410–3419 (2019).
Srinivas, N., Krause, A., Kakade, S. M. & Seeger, M. Gaussian process optimization in the bandit setting: no regret and experimental design. In Proc. 27th International Conference on Machine Learning (eds. Fürnkranz, J. & Joachims, T.) 1015–1022 (Omnipress, 2010).
Fox, R. et al. Optimizing the search algorithm for protein engineering by directed evolution. Protein Eng. 16, 589–597 (2003). This study is the first to use machine learning to guide directed evolution.
de Jong, S. Simpls: an alternative approach to partial least squares regression. Chemom. Intell. Lab. Syst. 18, 251–263 (1993).
The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 (2017).
Pan, S. J. & Yang, Q. A survey on transfer learning. IEEE Trans. Knowl. Data Eng. 22, 1345–1359 (2010).
Baker, D. An exciting but challenging road ahead for computational enzyme design. Protein Sci. 19, 1817–1819 (2010).
Radford, A., Metz, L. & Chintala, S. Unsupervised representation learning with deep convolutional generative adversarial networks. Preprint at https://arxiv.org/abs/1511.06434 (2015).
Ha, D. & Eck, D. A neural representation of sketch drawings. Sixth International Conference on Learning Representations https://openreview.net/forum?id=Hy6GHpkCW (2018).
Roberts, A., Engel, J., Raffel, C., Hawthorne, C. & Eck, D. A hierarchical latent vector model for learning long-term structure in music. Preprint at https://arxiv.org/abs/1803.05428 (2018).
Sinai, S., Kelsic, E., Church, G. M. & Nowak, M. A. Variational auto-encoding of protein sequences. Preprint at https://arxiv.org/abs/1712.03346 (2017).
Riesselman, A. J., Ingraham, J. B. & Marks, D. S. Deep generative models of genetic variation capture the effects of mutations. Nat. Methods 15, 816–822 (2018). This study predicts the effects of mutations without using any labeled data.
Kingma, D. P. & Welling, M. Auto-encoding variational Bayes. Preprint at https://arxiv.org/abs/1312.6114 (2014).
Costello, Z. & Garcia Martin, H. How to hallucinate functional proteins. Preprint at https://arxiv.org/abs/1903 (2019).
Müller, A. T., Hiss, J. A. & Schneider, G. Recurrent neural network model for constructive peptide design. J. Chem. Inf. Model. 58, 472–479 (2018).
Gupta, A. & Zou, J. Feedback GAN (FBGAN) for DNA: a novel feedback-loop architecture for optimizing protein functions. Preprint at https://arxiv.org/abs/1804.01694 (2018).
Anand, N. & Huang, P. Generative modeling for protein structures. In Advances in Neural Information Processing Systems 31 (eds. Bengio, S. et al.) 7504–7515 (Curran Associates, 2018).
Brookes, D. H. & Listgarten, J. Design by adaptive sampling. Preprint at https://arxiv.org/abs/1810.03714 (2018).
Brookes, D. H., Park, H. & Listgarten, J. Conditioning by adaptive sampling for robust design. Proc. Mach. Learn. Res. 97, 773–782 (2019).
Fowler, D. M. & Fields, S. Deep mutational scanning: a new style of protein science. Nat. Methods 11, 801–807 (2014).
Acknowledgements
The authors thank Y. Chen, K. Johnston, B. Wittmann, and H. Yang for comments on early versions of the manuscript, as well as members of the Arnold lab, J. Bois, and Y. Yue for general advice and discussions on protein engineering and machine learning. This work was supported by the US Army Research Office Institute for Collaborative Biotechnologies (W911F-09-0001 to F.H.A.), the Donna and Benjamin M. Rosen Bioengineering Center (to K.K.Y.), and the National Science Foundation (GRF2017227007 to Z.W.).
Author information
Authors and Affiliations
Contributions
K.K.Y., Z.W., and F.H.A. conceptualized the project. K.K.Y. wrote the manuscript with input and editing from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Nina Vogt was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, K.K., Wu, Z. & Arnold, F.H. Machine-learning-guided directed evolution for protein engineering. Nat Methods 16, 687–694 (2019). https://doi.org/10.1038/s41592-019-0496-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-019-0496-6
This article is cited by
-
Efficient evolution of human antibodies from general protein language models
Nature Biotechnology (2024)
-
Machine learning for functional protein design
Nature Biotechnology (2024)
-
Machine learning-guided engineering of genetically encoded fluorescent calcium indicators
Nature Computational Science (2024)
-
Opportunities and challenges in design and optimization of protein function
Nature Reviews Molecular Cell Biology (2024)
-
Effective engineering of a ketoreductase for the biocatalytic synthesis of an ipatasertib precursor
Communications Chemistry (2024)