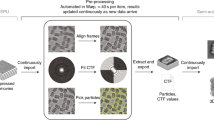
Abstract
The demand for high-throughput data collection in electron microscopy is increasing for applications in structural and cellular biology. Here we present a combination of software tools that enable automated acquisition guided by image analysis for a variety of transmission electron microscopy acquisition schemes. SerialEM controls microscopes and detectors and can trigger automated tasks at multiple positions with high flexibility. Py-EM interfaces with SerialEM to enact specimen-specific image-analysis pipelines that enable feedback microscopy. As example applications, we demonstrate dose reduction in cryo-electron microscopy experiments, fully automated acquisition of every cell in a plastic section and automated targeting on serial sections for 3D volume imaging across multiple grids.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All raw data presented in the ‘Applications’ section of this paper are available from the corresponding authors upon reasonable request.
References
Winey, M., Meehl, J. B., O’Toole, E. T. & Giddings, T. H. Jr. Conventional transmission electron microscopy. Mol. Biol. Cell 25, 319–323 (2014).
McMullan, G., Faruqi, A. R. & Henderson, R. Direct electron detectors. Methods Enzymol. 579, 1–17 (2016).
Frank, J. Advances in the field of single-particle cryo-electron microscopy over the last decade. Nat. Protoc. 12, 209–212 (2017).
Meijering, E., Carpenter, A. E., Peng, H., Hamprecht, F. A. & Olivo-Marin, J.-C. Imagining the future of bioimage analysis. Nat. Biotechnol. 34, 1250–1255 (2016).
Kreshuk, A., Koethe, U., Pax, E., Bock, D. D. & Hamprecht, F. A. Automated detection of synapses in serial section transmission electron microscopy image stacks. PLoS One 9, e87351 (2014).
Arganda-Carreras, I. et al. Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 33, 2424–2426 (2017).
Conrad, C. et al. Micropilot: automation of fluorescence microscopy–based imaging for systems biology. Nat. Methods 8, 246–249 (2011).
Neumann, B. et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464, 721–727 (2010).
Tan, Y. Z., Cheng, A., Potter, C. S. & Carragher, B. Automated data collection in single particle electron microscopy. Microscopy (Oxf.) 65, 43–56 (2016).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Rice, W. J. et al. Routine determination of ice thickness for cryo-EM grids. J. Struct. Biol. 204, 38–44 (2018).
Nicholson, W. V., White, H. & Trinick, J. An approach to automated acquisition of cryoEM images from lacey carbon grids. J. Struct. Biol. 172, 395–399 (2010).
Coudray, N. et al. Automated screening of 2D crystallization trials using transmission electron microscopy: a high-throughput tool-chain for sample preparation and microscopic analysis. J. Struct. Biol. 173, 365–374 (2011).
Hu, M. et al. Automated electron microscopy for evaluating two-dimensional crystallization of membrane proteins. J. Struct. Biol. 171, 102–110 (2010).
Cheng, A. Automation of data acquisition in electron crystallography. Methods Mol. Biol. 955, 307–312 (2013).
Gatel, C., Dupuy, J., Houdellier, F. & Hÿtch, M. J. Unlimited acquisition time in electron holography by automated feedback control of transmission electron microscope. Appl. Phys. Lett. 113, 133102 (2018).
Tejada, A., den Dekker, A. J. & Van den Broek, W. Introducing measure-by-wire, the systematic use of systems and control theory in transmission electron microscopy. Ultramicroscopy 111, 1581–1591 (2011).
Liu, J. et al. Fully mechanically controlled automated electron microscopic tomography. Sci. Rep. 6, 29231 (2016).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Berthold, M. R. et al. KNIME: the Konstanz Information Miner. In Data Analysis, Machine Learning and Applications (eds. Preisach, C. et al.) 319–326 (Springer, 2008).
Dietz, C. & Berthold, M. R. KNIME for open-source bioimage analysis: a tutorial. In Focus on Bio-Image Informatics (eds. Vos, W. H. D., Munck, S. & Timmermans, J.-P.) 179–197 (Springer, 2016).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
McQuin, C. et al. CellProfiler 3.0: next-generation image processing for biology. PLoS Biol. 16, e2005970 (2018).
Pau, G., Fuchs, F., Sklyar, O., Boutros, M. & Huber, W. EBImage—an R package for image processing with applications to cellular phenotypes. Bioinformatics 26, 979–981 (2010).
Prouteau, M. et al. TORC1 organized in inhibited domains (TOROIDs) regulate TORC1 activity. Nature 550, 265–269 (2017).
de Boer, P., Hoogenboom, J. P. & Giepmans, B. N. G. Correlated light and electron microscopy: ultrastructure lights up! Nat. Methods 12, 503–513 (2015).
Kukulski, W. et al. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J. Cell Biol. 192, 111–119 (2011).
Elserafy, M. et al. Molecular mechanisms that restrict yeast centrosome duplication to one event per cell cycle. Curr. Biol. 24, 1456–1466 (2014).
Marteil, G. et al. Over-elongation of centrioles in cancer promotes centriole amplification and chromosome missegregation. Nat. Commun. 9, 1258 (2018).
Bron, C. et al. Three-dimensional electron microscopy of entire cells. J. Microsc. 157, 115–126 (1990).
Mastronarde, D. N. & Held, S. R. Automated tilt series alignment and tomographic reconstruction in IMOD. J. Struct. Biol. 197, 102–113 (2017).
Quinn, T. A. et al. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc. Natl Acad. Sci. USA 113, 14852–14857 (2016).
Cruz-Roa, A. et al. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: application to invasive breast cancer detection. PLoS One 13, e0196828 (2018).
Tajbakhsh, N. et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans. Med. Imaging 35, 1299–1312 (2016).
Buchholz, T.-O., Jordan, M., Pigino, G. & Jug, F. Cryo-CARE: content-aware image restoration for cryo-transmission electron microscopy data. Preprint at https://arxiv.org/abs/1810.05420 (2018).
Cardona, A. et al. TrakEM2 software for neural circuit reconstruction. PLoS One 7, e38011 (2012).
Oliphant, T. E. Python for scientific computing. Comput. Sci. Eng. 9, 10–20 (2007).
van der Walt, S. et al. scikit-image: image processing in Python. PeerJ 2, e453 (2014).
Wood, C. et al. Collaborative computational project for electron cryo-microscopy. Acta Crystallogr. D Biol. Crystallogr. 71, 123–126 (2015).
Burnley, T., Palmer, C. M. & Winn, M. Recent developments in the CCP-EM software suite. Acta Crystallogr. D Struct. Biol. 73, 469–477 (2017).
McKinney, W. Data structures for statistical computing in Python. In Proc. 9th Python in Science Conference (eds. van der Walt, S. & Millman, J.) 51–56 (SciPy, 2010).
Anderson, J. R. et al. Exploring the retinal connectome. Mol. Vis. 17, 355–379 (2011).
Acknowledgements
We thank A. Krämer for initiating and coordinating the scientific project on leukocytes. We thank A. Desfosses and M. Prouteau for providing specimens for testing and application of the cryo-EM workflow. We acknowledge support from C. Palmer for mrcfile, and C. Dietz and C. von Schwerin for KNIME Image Processing and Python bindings. We thank all staff of the EM Core Facility at EMBL for helpful discussions and ideas. We thank R. Mellwig for critical reading of and comments on the manuscript. We also acknowledge support from the EMBL Center of Bioimage Analysis (CBA). I.H. is the recipient of an HRCMM (Heidelberg Research Center for Molecular Medicine) Career Development Fellowship. Work on SerialEM was supported originally by grants from NIH and more recently by contributions from users and from JEOL USA, Inc., as well as by payments for specific projects by Hitachi High Technologies America, Inc., JEOL USA, Inc., and Direct Electron, LP.
Author information
Authors and Affiliations
Contributions
M.S., I.H., W.J.H.H. and Y.S. designed the experimental applications. M.S. and D.N.M. did the software development. I.H., W.J.H.H. and M.S. collected the data. All authors contributed valuable suggestions on the necessary software functionality and edited the article. M.S. and D.N.M. wrote the initial manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
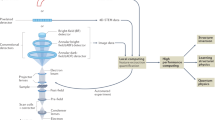
Supplementary Figure 1 The KNIME workflow used for detecting each cell on a resin section (Application 2).
a - the main KNIME workflow with nodes and data connectors. Red connections deliver parameters, grey connections indicate data (images, metadata) handover. Dark blue boxes show the configuration dialogs where the user provides the location of the Navigator file and some parameters for image analysis. The user can monitor the result of the segmentation using the "Interactive Segmentation View" or manually correct misplaced labels using the "Interactive Labeling Editor". The initial Python nodes shown on the bottom left use py-EM to parse the Navigator file and import the map image(s). If desired, an IMOD model can be drawn that will act as a mask defining the image area for processing. The image analysis meta-node contains the entire procedure that extracts the position and outline of each individual cell from the image. Its detailed contents are depicted in b. The "Interactive Labeling Editor" node enables manual curation of the detected cells. The final Python scripting node generates the Navigator file and fills it with a Virtual Map and the polygon outline for each cell. b - The KNIME image analysis pipeline executed within the meta-node shown in a. Each node performs an individual processing task. The respective result is shown above for reference. Some nodes incorporate external functionality from Python or ImageJ. The output is an image with a distinct pixel intensity value ("label") for each detected cell. The automated identification of cells presented in this figure has been successfully applied to 26 sections for this experiment and with modified image analysis pipelines for two different specimens.
Supplementary Information
Supplementary Information
Supplementary Figure 1
Supplementary Protocol 1
High-yield automated cryo-EM data acquisition of large particles
Supplementary Protocol 2
Automated serial-section TEM
Supplementary Video 1
Every detected cell on the section. An image stack with one overview image for each of the automatically detected 1,325 cells. Scale bar, 1 µm. Automated identification of cells as shown in this video has been successfully applied to 26 sections with about 1,000 cells each for this experiment, and with modified image-analysis pipelines for two different specimens
Supplementary Video 2
One cell across nine grids. Series of map images of a single cell of interest across 42 serial sections on 9 consecutive grids. The centriole is marked with a blue arrow. Scale bar, 1 µm. In the presented experiment, we followed a total of 120 cells across 100 sections on 20 grids for 3 different specimens
Supplementary Video 3
How to register serial sections. Video tutorial of the registration procedure from one section to the next. It demonstrates how to propagate the maps of 50 cells and relocate them on the next section
Supplementary Video 4
Reconstruction of serial tomograms of a centriole. Reconstruction of serial tomograms of the centriole region marked in SM1. The acquisition area spans six sections covering approximately 1.2 µm. Scale bar, 200 nm. In the presented experiment, we acquired a total of 992 tomograms in series on consecutive sections for 120 cells
Supplementary Software
Py-EM and SerialEM software files
Rights and permissions
About this article
Cite this article
Schorb, M., Haberbosch, I., Hagen, W.J.H. et al. Software tools for automated transmission electron microscopy. Nat Methods 16, 471–477 (2019). https://doi.org/10.1038/s41592-019-0396-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-019-0396-9
This article is cited by
-
Antigen self-anchoring onto bacteriophage T5 capsid-like particles for vaccine design
npj Vaccines (2024)
-
In situ structure of actin remodeling during glucose-stimulated insulin secretion using cryo-electron tomography
Nature Communications (2024)
-
Site-selective chlorination of pyrrolic heterocycles by flavin dependent enzyme PrnC
Communications Chemistry (2024)
-
Conformational ensemble of yeast ATP synthase at low pH reveals unique intermediates and plasticity in F1–Fo coupling
Nature Structural & Molecular Biology (2024)
-
Molecular mechanism of Oxr1p mediated disassembly of yeast V-ATPase
EMBO Reports (2024)