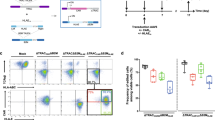
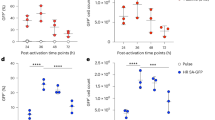
Abstract
Immune-cell engineering opens new capabilities for fundamental immunology research and immunotherapy. We developed a system for efficient generation of chimeric antigen receptor (CAR)-engineered T cells (CAR-T cells) with considerably enhanced features by streamlined genome engineering. By leveraging trans-activating CRISPR (clustered regularly interspaced short palindromic repeats) RNA (tracrRNA)-independent CRISPR–Cpf1 systems with adeno-associated virus (AAV), we were able to build a stable CAR-T cell with homology-directed-repair knock-in and immune-checkpoint knockout (KIKO CAR-T cell) at high efficiency in one step. The modularity of the AAV–Cpf1 KIKO system enables flexible and highly efficient generation of double knock-in of two different CARs in the same T cell. Compared with Cas9-based methods, the AAV–Cpf1 system generates double-knock-in CAR-T cells more efficiently. CD22-specific AAV–Cpf1 KIKO CAR-T cells have potency comparable to that of Cas9 CAR-T cells in cytokine production and cancer cell killing, while expressing lower levels of exhaustion markers. This versatile system opens new capabilities of T-cell engineering with simplicity and precision.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Code availability
Analytic codes used to generate figures that support the findings of this study will be made available by the corresponding author upon reasonable request.
Data availability
Genome sequencing data are available via SRA/BioProject under accession number PRJNA509600. Plasmids and libraries are being deposited to Addgene. A list of AAV vectors generated and used in this study is provided in Supplementary Table 2. Original and processed data are included in the figures, figure legends, and supplementary materials of this article. Other relevant data and materials that support the findings of this study will be made available by the corresponding author upon reasonable request.
References
Garfall, A. L. et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma. N. Engl. J. Med. 373, 1040–1047 (2015).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910 (2014).
Jackson, H. J., Rafiq, S. & Brentjens, R. J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
Labanieh, L., Majzner, R. G. & Mackall, C. L. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2, 377 (2018).
Rosenbaum, L. Tragedy, perseverance, and chance—the story of CAR-T therapy. N. Engl. J. Med. 377, 1313–1315 (2017).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011). 95ra73.
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Themis, M. et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol. Ther. 12, 763–771 (2005).
Howe, S. J. et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 118 3143–3150 (2008).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113 (2017).
Schumann, K. et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl. Acad. Sci. USA 112, 10437–10442 (2015).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405 (2018).
Bak, R. O., Dever, D. P. & Porteus, M. H. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 13, 358 (2018).
Ren, J. et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 8, 17002 (2017).
Liu, X. et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 27, 154 (2017).
Zetsche, B. et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 35, 31 (2017).
Kleinstiver, B. P. et al. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 34, 869 (2016).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015).
Li, B. et al. Engineering CRISPR-Cpf1 crRNAs and mRNAs to maximize genome editing efficiency. Nat. Biomed. Eng. 1, 0066 (2017).
Torikai, H. et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–5705 (2012).
Kim, H. et al. Surrogate reporters for enrichment of cells with nuclease-induced mutations. Nat. Methods 8, 941 (2011).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–856 (2018).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
Haso, W. et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20 (2018).
Levine, B. L., Miskin, J., Wonnacott, K. & Keir, C. Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev. 4, 92–101 (2017).
Ellis, J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 16, 1241–1246 (2005).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Dai, X. et al. Rapid modular CAR-T generation with CRISPR/Cpf1 and AAV systems. Protocol Exchange https://doi.org/10.1038/protex.2018.139 (2019).
Kochenderfer, J. N. et al. Construction and pre-clinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 32, 689 (2009).
Han, C. et al. Desensitized chimeric antigen receptor T cells selectively recognize target cells with enhanced antigen expression. Nat. Commun. 9, 468 (2018).
Chen, S. et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell 160, 1246–1260 (2015).
Wang, J. et al. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 44, e30 (2016).
Acknowledgements
We thank C. Fuchs and R. Herbst for their assistance and insightful discussions. We thank L. Ye, J. Li, L. Shen, M. Dong, R. Chow, Z. Bai, X. Zhang, and all other members of the Chen laboratory for technical assistance and discussions. We thank various colleagues in the Department of Genetics, Systems Biology Institute, Cancer Systems Biology Center, MCGD Program, Immunobiology Program, BBS Program, Cancer Center, and Stem Cell Center at Yale for assistance and/or discussion. We thank the Center for Genome Analysis, Center for Molecular Discovery, Pathology Tissue Services, Histology Services, High Performance Computing Center, West Campus Analytical Chemistry Core and West Campus Imaging Core, and Keck Biotechnology Resource Laboratory at Yale for technical support. S.C. is supported by the Yale SBI/Genetics Startup Fund, the Damon Runyon Dale Frey Award (grant/award number DFS-13-15), the Melanoma Research Alliance (412806, 16-003524), St-Baldrick’s Foundation (426685), the Breast Cancer Alliance, the Cancer Research Institute (CLIP), AACR (499395, 17-20-01-CHEN), the Mary Kay Foundation (017-81), the V Foundation (V2017-022), the Ludwig Family Foundation, the US Department of Defense (W81XWH-17-1-0235), the Sontag Foundation, the Chenevert Foundation, and the NIH/NCI (1DP2CA238295-01, 1R01CA231112-01, 1U54CA209992-8697, 5P50CA196530-A10805, 4P50CA121974-A08306). G.W. is supported by CRI Irvington and RJ Anderson postdoctoral fellowships. J.J.P. is supported by a Yale MSTP training grant from the NIH (no. T32GM007205).
Author information
Authors and Affiliations
Contributions
S.C. conceived the project. X.D. and S.C. designed the project. X.D. performed most experiments with the assistance of Y.D., H.R.K., G.W., and Y.E. J.J.P. developed computational pipelines and performed NGS data analysis. X.D., J.J.P., and S.C. prepared the manuscript. S.C. secured funding and supervised the work.
Corresponding author
Ethics declarations
Competing interests
This research is primarily supported by the Yale SBI/Genetics Startup Fund and the NIH/NCI. The funder has no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript. A provisional patent has been filed by S.C. at Yale University related to this study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 AAV–Cpf1-mediated TCR targeting in human primary CD4+ T cells.
a, Western blot showing the expression levels of LbCpf1 after electroporation with mRNA for 1–5 d (n = 1). b, Schematic of crTRAC design that targets the first exon of the TRAC gene. AAV9 was used for packaging. c, Quantitative results showing TCR knockout by AAV6–Cpf1 in a dose-dependent manner. Human primary CD4+ T cells infected with AAV6 MOI = 1e3 (n = 2), AAV6 MOI = 1e4 (n = 2) or AAV6 MOI = 1e5 (n = 5). Unpaired t test was used to assess significance. Vector versus 1e5 crTRAC, ***P < 0.001. Data are shown as mean ± s.e.m., plus individual data points on the bar graph. d, Quantification of TRAC indel frequency generated by AAV9–crTRAC with a titration series of MOI (n = 1).
Supplementary Figure 2 Indel analysis of AAV–Cpf1-mediated multiplexed gene editing in human primary CD4+ T cells.
a, T7E1 endonuclease assay for genome editing of both PDCD1 and TRAC loci in bulk or TCR– sorted T cells using AAV–crTRAC;crPDCD1 array with AAV9 packaging. Arrowheads point to cleavage products. b, Targeted mutagenesis efficiency of both PDCD1 and TRAC loci estimated by deep sequencing. The top ten most frequent variants at TRAC and PDCD1 loci generated by AAV9–crTRAC;crPDCD1, with the associated variant frequencies in the boxes to the right. Red arrowheads indicate predicted cleavage sites. One representative sample’s data are shown from 3 infection replicates. c, T7E1 endonuclease assay of genome editing of both PDCD1 and TRAC loci in bulk or TCR– sorted T cells using AAV–crTRAC;crPDCD1 array with AAV6 packaging. Arrowheads point to cleavage products. d, Targeted mutagenesis efficiency of both PDCD1 and TRAC loci estimated by deep sequencing. The top ten most frequent variants at TRAC and PDCD1 loci generated by AAV6–crTRAC;crPDCD1, with the associated variant frequencies in the boxes to the right. Red arrowheads indicate predicted cleavage sites. One representative sample’s data are shown from 3 infection replicates.
Supplementary Figure 3 Demonstration of one-step simultaneous generation of HDR-based transgene knockin at the TRAC locus with PDCD1 knockout in human primary CD4+ T cells.
a, Schematic of a PDCD1KO;dTomato-TRACKI AAV construct (TRAC-KIKO) and targeting strategy, where the dual crRNA array crTRAC;crPDCD1 generates double-strand breaks at both TRAC and PDCD1 loci, and an HDR donor (LHA-EFS-dTomato-PA-RHA) mediates dTomato integration into the TRAC locus. AAV6 was used for packaging. b, In-Out PCR for the targeted integration of dTomato at the TRAC locus. HDR and NHEJ products and percentage (quantified by gel-based method) are indicated by arrows pointing to bands of predicted sizes. c, The frequencies of dTomato HDR, TRAC NHEJ, and TRAC WT alleles in unsorted or CD3–dTomato+ sorted T cells, measured by Nextera prep and deep sequencing (n = 3 infection replicates). Two-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance. WT group: vector versus unsorted, ***P < 0.001; vector versus sorted, ***P < 0.001; unsorted versus sorted, **P < 0.01. NHEJ group: vector versus unsorted, P = 0.2201; vector versus sorted, P = 0.2547; unsorted versus sorted, P = 0.9952. HDR group: vector versus unsorted, ***P < 0.001; vector versus sorted, ***P < 0.001; unsorted versus sorted, **P < 0.01. Data are shown as mean ± s.e.m., plus individual data points on the graph. d, Example reads of HDR mapping with dTomato TRAC knockin reference sequence. e, The PDCD1 cutting efficacy in unsorted or CD3–dTomato+ sorted T cells, estimated by comparison to quantitative low-range DNA ladder as indicated below, and subsequently by using Nextera library preparation and Illumina sequencing. f, Indel analysis based on read-level quantification for PDCD1 knockout in unsorted or CD3–dTomato+ sorted T cells (n = 3 infection replicates). One-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance. Vector versus unsorted, ***P < 0.001; vector versus sorted, ***P < 0.001; unsorted versus sorted, **P < 0.01. Data are shown as mean ± s.e.m., plus individual data points on the graph.
Supplementary Figure 4 Quantification for modular combinations of AAV–Cpf1-mediated efficient multiplex knockin and knockout in human primary CD4+ T cells.
a, Quantification of dTomato-knockin frequency at TRAC target sites shown in a dot-plot graph (uninfected n = 2 cell culture replicates; AAV vector n = 3 infection replicates; PDCD1KO;dTomato-TRACKI n = 3 infection replicates). AAV vector sample was infected only with AAV6 carrying the same construct, and did not receive electroporation of Cpf1 thereafter. Unpaired two-sided t test was used to assess significance. Vector versus TRAC-KIKO, **P < 0.01. Data are shown as mean ± s.e.m., plus individual data points on the graph. b, Quantifications of GFP-knockin frequency at PDCD1 target site shown in a dot-plot graph (cell culture or infection replicates, n = 6). Unpaired t test was used to assess significance. Vector versus PDCD1-KIKO, ***P < 0.001. Data are shown as mean ± s.e.m., plus individual data points on the graph. c, Quantification of percentages of GFP+ and dTomato+ cells shown in a dot-plot graph with one vector system (infection replicates, AAV vector n = 3; dTomato-TRACKI;GFP-PDCD1KI n = 4). Unpaired two-sided t test was used to assess significance. Vector versus dTomato-TRACKI;GFP-PDCD1KI, ***P < 0.001 for all knockin comparisons (GFP+, dTomato+ and GFP+dTomato+). Data are shown as mean ± s.e.m., plus individual data points on the graph. d, Quantification of percentages of GFP+ and dTomato+ cells shown in a dot-plot graph with two-vector system (infection replicates, n = 3). Unpaired two-sided t test was used to assess significance. Vector versus dual targeting, ***P < 0.001 for all knockin comparisons (GFP+, dTomato+ and GFP+dTomato+). Data are shown as mean ± s.e.m., plus individual data points on the graph.
Supplementary Figure 5 Cell viability test of the AAV–Cpf1 system.
a, Estimation of cells lost because of electroporation, or because of the combined effects of electroporation and AAV infection for 24 h (cell culture replicates, n = 3). b, Live cell percentage before and after electroporation along a time course, in which viability was determined by trypan blue staining. Cell viability showed no significant difference before and after electroporation across all time points. Kruskal–Wallis test was used to assess statistical significance (P = 0.0587) (cell culture replicates, n = 3). Data are shown as mean ± s.e.m. c, Engineered CD22BBz KIKO CAR-T cell growth time course, with T cells stimulated with mitomycin-C-pretreated NALM6 cells at a 1:1 ratio each week. The total number of cells is shown along different time points for a maximum of 26 d. Arrows indicate stimulation time points (cell culture replicates, n = 3). Data are shown as mean ± s.e.m.
Supplementary Figure 6 Additional supporting data for high-efficiency generation of stably integrated anti-CD22 CAR-T cells with PDCD1 knockout by AAV–Cpf1 KIKO.
a, Quantitative results of FACS showed that AAV–Cpf1 yielded a knockin frequency of 44.6% in human primary CD4+ T cells in one transduction (n = 3 infection replicates). Unpaired two-sided t test was used to assess significance. Vector versus CD22BBz KIKO, ***P < 0.001. Data are shown as mean ± s.e.m., plus individual data points on the bar graph. b, In-Out PCR for targeted integration of CD22BBz CAR at TRAC locus. HDR and NHEJ products are indicated by arrows pointing to bands of predicted sizes. c, Example reads of HDR mapping with CD22BBz CAR TRAC knockin reference sequence in contrast to wild-type genomic locus. d, The PDCD1 knockout in unsorted or CD3–CD22CAR+ sorted T cells identified by T7E1 assay. Arrowheads point to cleavage products. e, Representative flow cytometry histogram showing the CAR expression on CD22BBz KIKO after transduction for 3, 5, 7, and 9 d. One representative case is shown from n = 3 infection replicates.
Supplementary Figure 7 Modular combinations of AAV–Cpf1-mediated efficient dual-CAR knockin and knockout in human primary CD4+ T cells.
a, Schematics of construct design for TRACKO;CD19BBz-PDCD1KI (CD19BBz-KIKO) for AAV–Cpf1mediated CD19BBz transgene knockin into PDCD1 locus with TRAC knockout. b, Representative flow cytometry plots showing PDCD1 CD19BBz knockin 8 d after AAV transduction (AAV6 packaging). c, Quantification of CD19BBz knockin frequency at PDCD1 target site shown in a column graph (uninfected n = 2 cell culture replicates; AAV vector n = 3 infection replicates; TRACKO;CD19BBz-PDCD1KI n = 3 infection replicates). Unpaired two-sided t test was used to assess significance. Vector versus CD19BBz-KIKO, ***P < 0.001. Data are shown as mean ± s.e.m., plus individual data points on the bar graph. d, Schematics of construct design for using both PDCD1KO;CD22BBz-TRACKI and TRACKO;CD19BBz-PDCD1KI for dual targeting. e, Representative flow cytometry plots of double knockin 5 d after transduction for both AAV constructs (AAV6 packaging). f, Quantification of percentages of CD19BBz and CD22BBz single- and double-positive cells shown in a dot-plot graph (infection replicates, n = 3). Two-way ANOVA with Sidak’s multiple-comparisons test was used to assess significance (multiple-testing corrected). Vector versus dual targeting, ***P < 0.001. Data are shown as mean ± s.e.m., plus individual data points on the graph. g, Analysis of TCR knockout at integrated cells by FACS. Quantification of TCR– cells in different quadrants shown in a dot-plot graph (infection replicates, n = 3). Unpaired two-sided t-test was used to assess significance. TCR– population, vector versus dual targeting, **P < 0.01, ***P < 0.001. Data are shown as mean ± s.e.m.
Supplementary Figure 8 AAV–Cas9 RNP–mediated single-CAR knockin and knockout in human primary CD4+ T cells with different guide RNAs.
a, Representative flow cytometry plots of TRAC CD22BBz knockin after 5 d using TRAC-sg1 and PDCD1-sg2 (AAV6 packaging). b, Quantification of CD22BBz knockin frequency at TRAC target site using TRAC-sg1 and PDCD1-sg2/sg3/sg4 5 d after AAV transduction (infection replicates, AAV vector n = 4; TRAC sg1 + PDCD1 sg2 n = 6; TRAC sg1 + PDCD1 sg3/sg4 n = 3). AAV vector sample was infected only with AAV6 carrying the same construct, and did not receive electroporation of Cas9 RNP thereafter. One-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance. Vector versus CD22BBz KIKO, ***P < 0.001. c, Representative flow cytometry plots showing PDCD1 CD19BBz knockin after 5 d with TRAC-sg1 and PDCD1-sg2 (AAV6 packaging). d, Quantification of CD19BBz knockin frequency at TRAC target site using TRAC-sg1 and PDCD1-sg2/sg3/sg4 5 d after AAV transduction (infection replicates, AAV vector n = 4; TRAC sg1 + PDCD1 sg2 n = 6; TRAC sg1 + PDCD1 sg3/sg4 n = 3). One-way ANOVA with Tukey’s multiple comparisons test was used to assess significance. Vector versus TRAC sg1+ PDCD1 sg2, ***P < 0.001. Vector versus TRAC sg1 + PDCD1 sg3, n.s. P = 0.1331. Vector versus TRAC sg1+ PDCD1 sg4, *P < 0.05. Data are shown as mean ± s.e.m., plus individual data points on the bar graph.
Supplementary Figure 9 Quantification of AAV–Cpf1 and Cas9 RNP-based double-CAR knockin.
a, FACS quantification of percentages of CD19CAR and CD22CAR single- and double-positive cells shown in a dot-plot graph (infection replicates, n = 3). Two-way ANOVA with Sidak’s multiple-comparisons test was used to assess significance (multiple-testing corrected). Vector versus Cpf1 CAR19;CAR22 double knockin: for CAR19+ cells, ***P < 0.001; for CAR22+ cells, ***P < 0.001; for CAR19+CAR22+ cells, ***P < 0.001. b, FACS quantification of percentages of CD19CAR and CD22CAR single- and double-positive cells shown in a column graph (infection replicates, vector n = 4; Cas9 CAR19;CAR22 double knockin, n = 6). Two-way ANOVA with Sidak’s multiple-comparisons test was used to assess significance (multiple-testing corrected). Vector versus Cas9 CAR19;CAR22 double knockin: for CAR19+ cells, ***P < 0.001; for CAR22+ cells, n.s. P = 0.8036; for CAR19+CAR22+ cells, n.s. P = 0.9084. c, Quantification of percentages of AAV–Cas9-mediated double knockin into TRAC and PDCD1 locus using TRAC-sg1 and PDCD1-sg2/sg3/sg4 (infection replicates, vector n = 4, TRAC sg1 + PDCD1 sg2 n = 6 and TRAC sg1 + PDCD1 sg3/sg4 n = 3). Two-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance (multiple-testing corrected). CAR19+ group: vector versus TRAC sg1 + PDCD1sg2/ sg3/sg4, ***P < 0.001. CAR22+ group: vector versus PDCD1-sg2, P = 0.678; vector versus PDCD1-sg3, P = 0.824; vector versus PDCD1-sg4, P = 0.885. CAR19+CAR22 + group: vector versus PDCD1-sg2, P = 0.439; vector versus PDCD1-sg3, P = 0.684; vector versus PDCD1-sg4, P = 0.493. Data are shown as mean ± s.e.m., plus individual data points on the graph.
Supplementary Figure 10 NALM6 CD22 and CD19 expression and NALM6-GL cell line generation.
a, Flow cytometry analysis of CD22 and CD19 expression in the NAML6 cell line. b, NALM6 cells were infected with a lentivirus that constitutively expresses GFP and luciferase reporter genes. After infection for 2 d, GFP+ cells were sorted by FACS. c, The expression of luciferase in NALM6-GL cells was confirmed by luciferase imaging using IVIS.
Supplementary Figure 11 Functional assessment of Cpf1 double-knockin KIKO CAR-T cells.
a, Cytolytic activities of CAR22 (CD22BBz), CAR19 (CD19BBz) and CAR22;CAR19 (CD22BBz;CD19BBz double knockin) KIKO CAR-T cells 5 d after electroporation and AAV infection were tested by in vitro coculture (kill assay). In vitro cytotoxic activity was measured by bioluminescence assay at different effector:target (E:T) ratios, for AAV-vector transduced T cells, CAR22, CAR19 and CAR22;CAR19 double knockin KIKO CAR-T cells (n = 3 infection replicates for each experiment condition). Two-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance. Vector versus KIKO CAR-T cell, ***P < 0.001 for all three groups (CAR22, CAR19 and CAR22;CAR19); CAR22 versus CAR19, P = 0.9994; CAR22 versus CAR22;CAR19, P = 0.9997; CAR19 versus CAR22;CAR19, P > 0.9999. b, Representative flow cytometry results showing IFN-γ and TNF-α expression levels in CAR22, CAR19 and CAR22;CAR19 T cells. IFN-γ and TNF-α production was tested by intracellular staining after coculture with NALM6 cells for 5 h at E:T = 1:1. One representative case is shown from n = 3 infection replicates. c, Quantitative results demonstrating the antigen-specific IFN-γ and TNF-α production capability of CAR22, CAR19, and CAR22;CAR19 T cells (n = 3 infection replicates). Two-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance (multiple-testing corrected). IFN-γ group: vector versus CAR22, ***P < 0.001; vector versus CAR19, ***P < 0.001; vector versus CAR22;CAR19, ***P < 0.001; CAR22 versus CAR19, ***P < 0.001; CAR22 versus CAR22;CAR19, ***P < 0.001; CAR19 versus CAR22;CAR19, ***P < 0.001. TNF-α group: vector versus CAR22, ***P < 0.001; vector versus CAR19, ***P < 0.001; vector versus CAR22;CAR19, ***P < 0.001; CAR22 versus CAR19, P = 0.4499; CAR22 versus CAR22;CAR19, ***P < 0.001; CAR19 versus CAR22;CAR19, **P = 0.0021. All data are shown as mean ± s.e.m., plus individual data points on the graph.
Supplementary Figure 12 Quantitation of cytokine and T cell exhaustion markers of Cpf1 CD22BBz KIKO CAR-T cells compared to Cas9-mediated CD22BBz;PDCD1–/– CAR-T cells.
a, FACS quantitative analysis of antigen-specific IFN-γ and TNF-α production capability in Cpf1 CD22BBz KIKO versus Cas9 RNP CD22BBz CAR-T cells (n = 3 infection replicates each). Two-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance (multiple-testing corrected). IFN-γ group: vector versus Cas9, ***P < 0.001; vector versus Cpf1, ***P < 0.001; Cas9 versus Cpf1, P = 0.4835. TNF-α group: vector versus Cas9, ***P < 0.001; vector versus Cpf1, ***P < 0.001; Cas9 versus Cpf1, P = 0.1318. b, FACS quantitative analysis of Cpf1 CD22BBz KIKO versus Cas9 RNP CD22BBz CAR-T cells in T cell exhaustion, showing that the expression of exhaustion markers (PD-1, TIGIT, and LAG3) in Cpf1 CD22BBz KIKO CAR-T cells was lower than that in Cas9 RNP CD22BBz CAR-T cells (n = 3 infection replicates each). Two-way ANOVA with Tukey’s multiple-comparisons test was used to assess significance (multiple-testing corrected). PD-1 group: vector versus Cas9, ***P < 0.001; vector versus Cpf1, P = 0.9087; Cas9 versus Cpf1, ***P < 0.001. TIGIT group: vector versus Cas9, ***P < 0.001; vector versus Cpf1, ***P < 0.001; Cas9 versus Cpf1, ***P < 0.001. LAG3 group: vector versus Cas9, ***P < 0.001; vector versus Cpf1, ***P < 0.001; Cas9 versus Cpf1, ***P < 0.001. All data are shown as mean ± s.e.m., plus individual data points on the graph.
Supplementary Figure 13 FACS gating.
A representative figure of FACS gating.
Supplementary information
Supplementary Information
Supplementary Figures 1–13
Supplementary Protocol
Rapid modular CAR-T generation with CRISPR/Cpf1 and AAV systems
Supplementary Table 1
Construction of AAV vectors.
Supplementary Table 2
List of constructs.
Supplementary Data 1
Indel variant statistics.
Rights and permissions
About this article
Cite this article
Dai, X., Park, J.J., Du, Y. et al. One-step generation of modular CAR-T cells with AAV–Cpf1. Nat Methods 16, 247–254 (2019). https://doi.org/10.1038/s41592-019-0329-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-019-0329-7
This article is cited by
-
Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma
Virology Journal (2024)
-
Expanded Alternatives of CRISPR–Cas9 Applications in Immunotherapy of Colorectal Cancer
Molecular Diagnosis & Therapy (2024)
-
In vivo manufacture and manipulation of CAR-T cells for better druggability
Cancer and Metastasis Reviews (2024)
-
Homology-independent targeted insertion (HITI) enables guided CAR knock-in and efficient clinical scale CAR-T cell manufacturing
Molecular Cancer (2023)
-
AAV-mediated delivery of a Sleeping Beauty transposon and an mRNA-encoded transposase for the engineering of therapeutic immune cells
Nature Biomedical Engineering (2023)